- Open-Access Publishing
- Quality and Potential Expertise
- Flexible Online Submission
- Affordable Publication Charges
- Expertise Editorial Board Members
- 3 Week Fast-track Peer Review
- Global Visibility of Published Articles
Long-term Results of Stentless Bio-Conduits for Bentall Operation
Guglielmo Stefanelli1*, Fabrizio Pirro1, Fabio Sgura3, Antonio D’Adamo1, Luca Weltert2
1Department of Cardiac Surgery and Cardiology, Hesperia Hospital, Modena, Italy
2Department of Cardiac Surgery, European Hospital, Rome, Italy
3Department of Cardiology, University Hospitals, Modena, Italy
*Corresponding Author: Guglielmo Stefanelli, Department of Cardiac Surgery and Cardiology, Hesperia Hospital, Modena, Italy, E-mail: guglielmostefanelli@gmail.com
Received date: July 07, 2021; Accepted date: August 02, 2021; Published date: August 09, 2021
Citation: Stefanelli G, Pirro F, Sgura F, D’Adamo A, Weltert L. (2021) Long-term Results of Stentless Bio-Conduits for Bentall Operation. J Card Cardi Sur. 1:08.
Copyright: © 2021 Stefanelli G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Aortic root surgery; Bentall operation; Stentless aortic valve
Abstract
Background: To evaluate the early and long-term clinical outcomes of patients undergoing Bentall operation by stentless bio-conduits for aortic root dilatation associated with aortic valve disease.
Methods: From 2004 to 2015 a cohort of 56 patients underwent Bentall operation to replace the aortic root and the aortic valve at our unit. Mean age at operation was 67 ± 9.63 years. The first 30 patients received a Shelhigh NR-2000 bioprosthesis, in the last 26 cases a self-assembled Medtronic 3F bio-root was implanted. Mean. Euroscore was 13.30 ± 9.91, 14 patients (25%) underwent associated procedures. In 6 cases (10.91%) the aortic valve was bicuspid. Five patients (9.09%) had undergone a previous cardiac operation. Mean follow-up time was 7.09 years (0.26-14.50 years) and it was 100% complete. Patients were evaluated and analyzed for intra-operative results and long-term clinical outcomes.
Results: Peri-operative/in hospital mortality was 3.64% (2 patients). Overall survival probability at 11 years was 74.9%. 5 patients (9.09%) died during follow-up for cardiac reasons. Freedom from MACCE at 11 years was 81.3%. Freedom from structural valve deterioration at 11 years was 91.6%. Freedom from reoperation at follow- up was 97.9%. Mean gradient at last follow-up was 11.70 mmHg.
Conclusion: Bentall procedure using stentless bio-conduits achieves excellent results at long -time follow-up. The haemodynamic performance is adequate and preserved over time, making this prosthesis a good choice for younger, active patients
Background
Bentall operation was conceived and first reported in late 1960s [1]. The procedure was upgraded several years later by Kochoukos et al. [2], who developed the ‘button-Bentall’ modification, which still remains the standard surgical procedure for patients with dilated aortic root associated to aortic valve pathology. As happened for isolated aortic valve replacement, and mainly due to the increasing patient’s demand for biological devices, almost all Bentall operations are nowadays accomplished using a valved bio-conduit. Several pre-assembled prostheses, consisting of a tubular straight or Valsalva graft including a mechanical or biological valve are available on the market. In addition, self-assembled devices, obtained by inserting at the operative table a stented bioprosthesis into a fabric graft, represent a valid alternative to ready-to-use conduits, and are widely in use. In this study we report the long-term outcomes of patients undergoing a full-stentless self-made bio-root implantation. In the first part of our experience we utilized the ready-to-use Shelhigh NR-2000 prosthesis, a commercial full-biological composite xeno-pericardial tubular graft containing a stentless porcine valve, while the last 30 patients received a self-made bio-conduit, assembled by including a stentless Medtronic 3F equine pericardial valve into a straight woven fabric tube graft. By using a stentless bio-conduit, instead of a stented one, the haemodynamic behaviour of the prosthesis it is expected to improve, particularly under exercise, and its durability could be extended.
Material and Methods
Population
Between 2004 and 2015 a cohort of 56 patients underwent replacement of the aortic root for dilatation of the ascending aorta associated to aortic valve disease at our unit by a single surgeon. Patients without significant (>45 mm) aortic root dilatation were excluded from the study. All patient received routine pre-operative evaluation by echocardiography and computed tomography, along with coronary angiogram. The study was approved by the Institutional Ethical Committee. Preoperative, intra-operative and post-operative data were collected from the hospital medical records and informed consent to use their data was obtained from all the patients. Guidelines reported by Akins et al were used to define the endpoints of the analysis.
Mean age at surgery for the entire cohort was 67 ± 9.68 years. 40 patients (74.10%) were males. Table 1a reassumes the pre-operative patient’s details. The mean logistic EuroSCORE was 12.98 ± 9.95, quite high, with 27.80% of patients being in NYHA class III-IV. Mean Ejection Fraction (EF) was 57 ± 7.62%. Mean Left Ventricular End-Diastolic Diameter (LVEDD) was 54 ± 8.50 mm. A Bicuspid Aortic Valve (BAV) was present in 6 patients (11.10%). According to Sievers calssification, in 2 cases the BAV was type 0, without rafe, in 3 cases was type 1/L-R, in one case was type 1/R-N. Four patients (7.40%) had undergone a previous open heart operation.
| BIO-BENTALL | ||
| Mean/n° | St. Dev/% | |
| AGE (years) | 67 | 9.68 |
| Gender (Male) | 40 | 74.10% |
| BSA (m2) | 1.82 | 19.00% |
| NYHA III-IV | 15 | 27.80% |
| Log Euroscore % | 12.98 | 995.00% |
| EF_Pre-op % | 57 | 7.62 |
| LVEDD_Pre-op (mm.) | 54 | 8.5 |
| BAV | 6 | 11.10% |
| Aortic. Dissection | 0 | 0.00% |
| Marfan Disease | 0 | 0.00% |
| ABE_Pre | 0 | 0.00% |
| REDO_pre | 4 | 7.40% |
| Abbreviations: BSA: Body Surface Area; NYHA: New York Heart Association; EF: Ejection Fraction; LVEDD: Left Ventricular End-Diastolic Diameter; BAV: Bicuspid Aortic Valve; AO: aortic; ABE: Acute Bacterial Endocarditis. | ||
Table 1a: Baseline patient's characteristics.
Surgical technique
Routine coronary angiography along with echocardiographic evaluation was carried out in all patients before operation. The ‘button Bentall’ technique, as originally described by Kouchoukos was adopted in all cases, regardless of the type of prosthesis. All operations were performed by median sternotomy, standard Extra-Corporeal Circulation (ECC) at 34°C, Aortic Cross-Clamping (ACC) and cold cardioplegic arrest (CCA), by antegrade or retrograde infusion of Custodiol® solution. Cardiocirculatory arrest at 25°C and brain protection by selective perfusion of neck vessels was adopted in cases of aortic dissection or aortic arch replacement. After transverse aortotomy and excision of the aortic wall and of the native aortic valve, the two coronary buttons were prepared. In cases of calcified aortic annulus accurate debridement was carried out. The proximal side of the graft was secured to the left ventricular outflow by interrupted pledgedet mattress 2-0 braided polyester sutures, taking care not to shrink the aortic annulus. In all cases receiving a Shelhigh prosthesis a single (or sometime a triple) continuous 2-0 polypropylene suture, reinforced with a strip of autologous pericardium was chosen.
Next, the coronary ostia were reimplanted on the prosthesis using continuous 5-0 polypropylene sutures, and finally the graft was anastomosed to the distal aorta by a continuous 4-0 polypropylene suture reinforced by a teflon strip. In patients selected for a 3F Bio-root implant, the conduit was self-assembled by the surgeon at the back table before ECC start. As a first step the tube graft size was selected, based on accurate intra-operative transesophageal echo (TEE) measurements of aortic annulus and ventricular-aortic junction. In cases of calcified aortic valve three sizes were added to the calculated one. As a result, 28 mm or 30 mm have been the two graft sizes chosen for all patients, selecting a 3F valve one mm. smaller than the tubular prosthesis. After valve inclusion into the graft, three 4-0 polypropylene continuous running sutures secured the prosthesis cuff to the graft and the folded graft skirt was straighten up. Next, the three prosthetic commissural tabs were positioned and attached to the inner graft by three 4-0 polypropylene stitches, positioned at 120° (Figure 1). In case of future prosthetic replacement for Structural Valve Deterioration (SVD), only the valve leaflets removal would be necessary, using the prosthetic sewing cuff as a support for the new device implant, thus avoiding a new Bentall procedure. For the first days after surgery a dosage of low-molecular-weight heparin was given to all patients, followed by administration of low-dosage aspirin and statins for one year after hospital discharge. Oral anticoagulation with vitamin K antagonist was chosen for patients with history of atrial fibrillation or receiving an associated procedure involving the mitral valve.
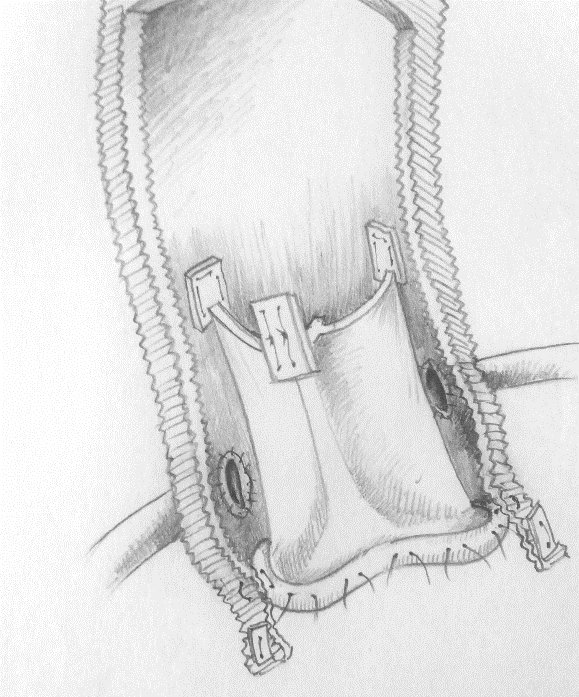
Figure 1: 3F Bioroot assembled and implanted.
Statistical analysis
Categorical variables were presented as numbers and percentages and were analyzed by Pearson’s χ2 or Fisher’s exact test. Continuous variables were expressed as mean ± standard deviation (SD) or median with interquartile range. Normality of the data was assessed using Shapiro-Wilk test. Differences between groups were compared using the Student's test for normally distributed continuous variables while for non-normally distributed continuous variables the Mann-Whitney U test was used. Kaplan. Meier method was used to plot graphs, with confidence intervals drawn as thinner lines for sake of visual clarity, number at risk is plotted in the lower row, all curves are cut when the 10% of total patient at risk is reached. Cox regression is used to test the impact of different covariates on study endpoint. P-values of <0.05 were considered statistically significant. All statistical analyses were performed using JMP® Version 13.1.0 and SAS 9.4 (SAS Institute Inc.).
RESULTS
Early Results
Associated procedures were carried out in 14 patients (25.90%). 7 patients (13.0%) received a concomitant coronary artery bypass graft (CABG). 5 patients (9.1%) underwent mitral valve repair, 2 patients (3.6%) required total aortic arch replacement. The mean aortic graft size was 27.63 ± 2.20 mm. Previous cardiac surgical procedures had been performed in 4 patients (7.40%). An Haemashield straight vascular prosthesis (Maquet, Rastatt, Germany) was used in the 30 patients receiving the self-assembled 3F bio-conduit. In the two cases requiring aortic arch replacement, a Shelhigh pericardial valved conduit was used for Bentall procedure and concomitant aortic arch reconstruction. Mean CPB time was 138 ± 61’ Mean ACC time was. 106 ± 32’. Re-exploration for bleeding was necessary in 4 patients (7.40%). Length of stay in Intensive Care Unit (ICU LOS) was 34 ± 24 hours. Permanent pacemaker (PMK) implantation was necessary in 1 patient. Peak transvalvular aortic gradient evaluated by echocardiography at hospital discharge was 18.75 ± 5.71 mmHg. Mean transvalvular gradient was 11.04 ± 6.82 mmHg. Data are illustrated in Table 1b.
| BIO-BENTALL | ||
| Mean/n° | St. Dev/% | |
| Assoc. Proc. | 14 | 25.90% |
| CABG | 7 | 13.00% |
| Mitral Valve Repair | 5 | 9.09% |
| Aortic Arch Replac. | 2 | 3.60% |
| Aortic Graft_Size (min.) | 27.63 | 2.2 |
| ECC | 138 | 61 |
| AXCL (min.) | 106 | 32 |
| ICU_LOS | 34 | 24 |
| Re Expl. For Bleeding | 4 | 7.40% |
| Deep Sternal Infection | 1 | 1.85% |
| PMK implant | 1 | 1.85% |
| Acute Renal Failure | 0 | 0.00% |
| HOSP death | 0 | 0.00% |
| ΔPmax DIS (mmHg.) | 18.55 | 5.71 |
| ΔPmed DIS (mmHg.) | 10.88 | 6.82 |
| AOI trivial to mild DIS | 4 | 7.40% |
| Abbreviations: CABG: Coronary Artery Bypass Graft; ECC: extracorporeal circulation; AXCL: aortic cross clamping; ICU LOS: Intensive Care Unit Length Of Stay; PMK: pace-maker; ΔP: left-ventricle-aortic gradient; AOI: aortic valve insufficiency; Dis: Discharge. | ||
Table 1b: Peri-operative and hospital discharge patient’s characteristics.
Early perioperative/in hospital mortality was 3.64% (2 patients).
A 72 years old woman with acute aortic dissection presented with a complete detachment of the right coronary ostium and massive infarction of the right ventricle. After replacement of the aortic arch on cardiocirculatory arrest and a Bentall operation with reimplantation of the right coronary ostium by a short autologous saphenous vein graft interposition, she died on the next day because of intractable right ventricular failure related to pre-operative myocardial infarction. The second patient, a 75 years old female, operated 5 years before for acute aortic dissection, presented with a devastating ABE involving the ascending aortic prosthesis, with complete dehiscence of the proximal and distal anastomosis and substernal abscess. The operation consisted of complete replacement of the dissected aortic arch with descending aortic fenestration on cardiocirculatory arrest started before sternotomy, selective cerebral antegrade protection, and replacement of the ascending aorta and aortic root by Bentall procedure. The patient died a few hours after surgery for intractable sudden mediastinal bleeding.
Late results
The median follow-up time was 7.161 years (range: 5 months-14.50 years), corresponding to 352 pts/years, and was 100% complete. To gain all follow-up data, all patients were contacted by the same researcher using a brief questionnaire and echo data and clinical examinations were gathered from the patient’s own cardiologist or from referring hospitals.
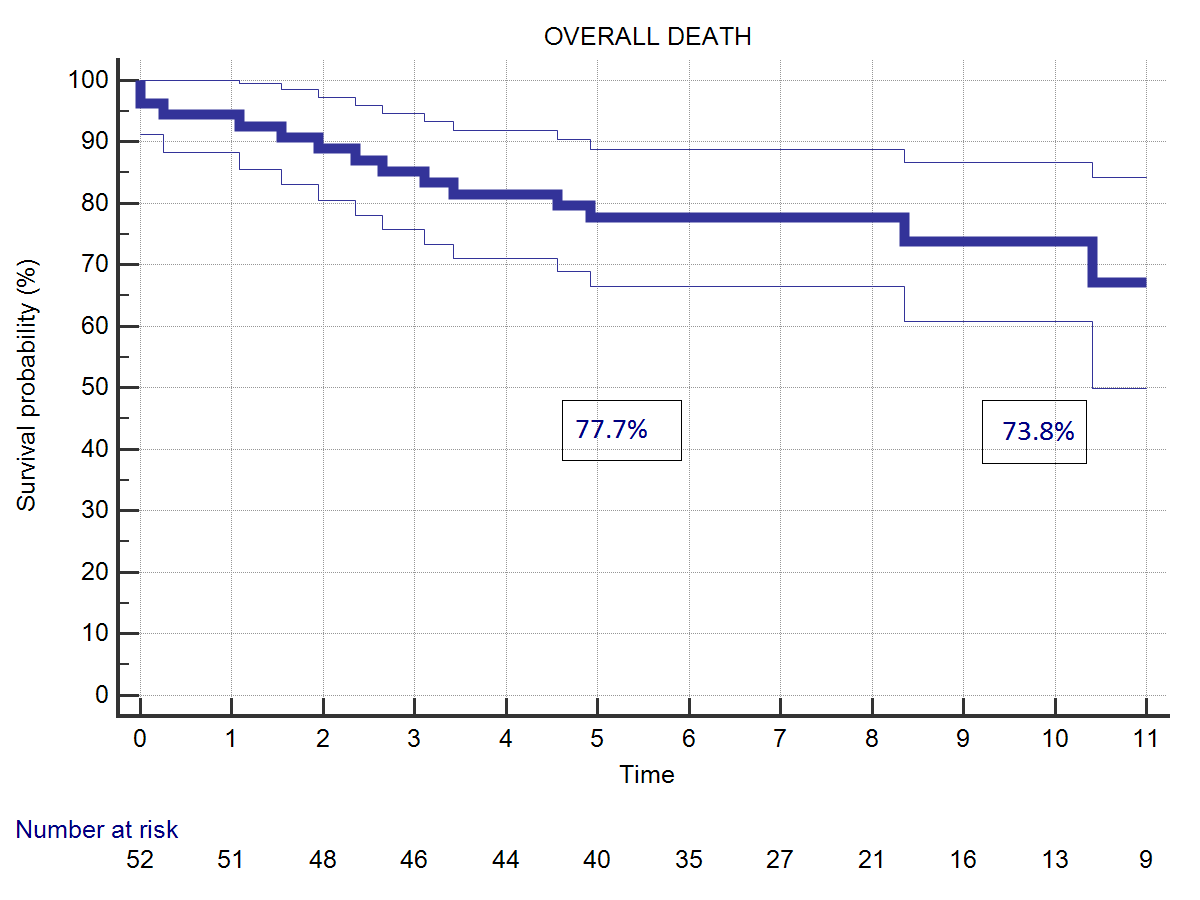
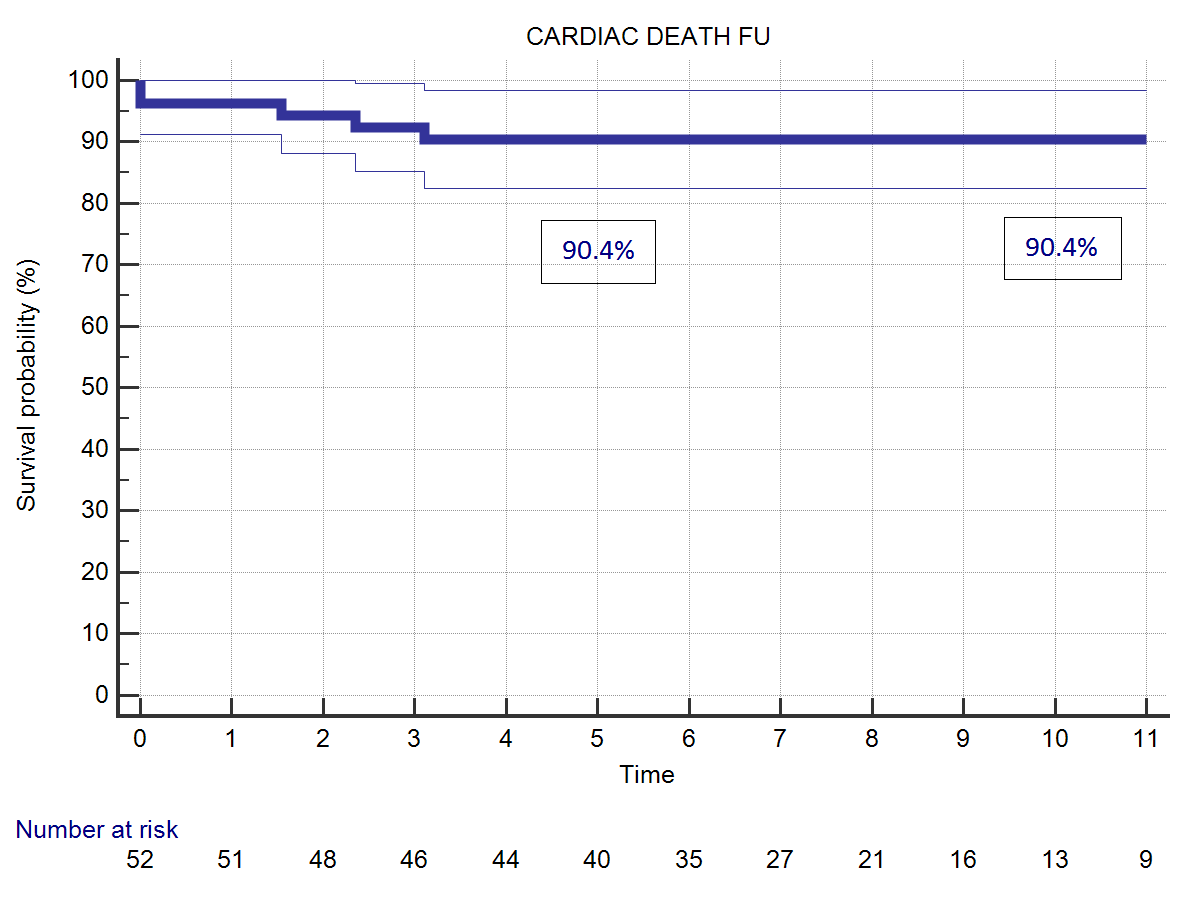
Twelve patients (22.2%) died during follow-up time. One patient died for acute myocardial infarction ten years after the operation. Another patient died for acute aortic dissection three years later. A patient with uncontrolled diabetes died few months after the operation due to severe acute unrecognized hypoglycemia. Nine patients died for non-cardiac causes, mostly because of cancer or aging. Five patients died during follow-up for cardiac causes (9.30%). Overall actuarial survival probability was 77.7% at 5 years and 73.8% at 11 years (Figure 2). Freedom from cardiac related mortality at 5 and 11 years was 90.4%. Logistic EuroSCORE (p<0.0001), Body Surface Area (BSA) (0.038), and NYHA class at follow-up (p<0.0001) were identified risk factors for late cardiac and non-cardiac death, Age at operation (p=0.0151) and concomitant mitral surgery (p=0.0146) resulted risk-factors for overall death. Duration of CPB was an additional risk-factor for late cardiac mortality (p=0.0004).
 |  |
| (A) | (B) |
| Figure 2: (A) Overall survival probability. (B) Survival probability related to cardiac deaths. | |
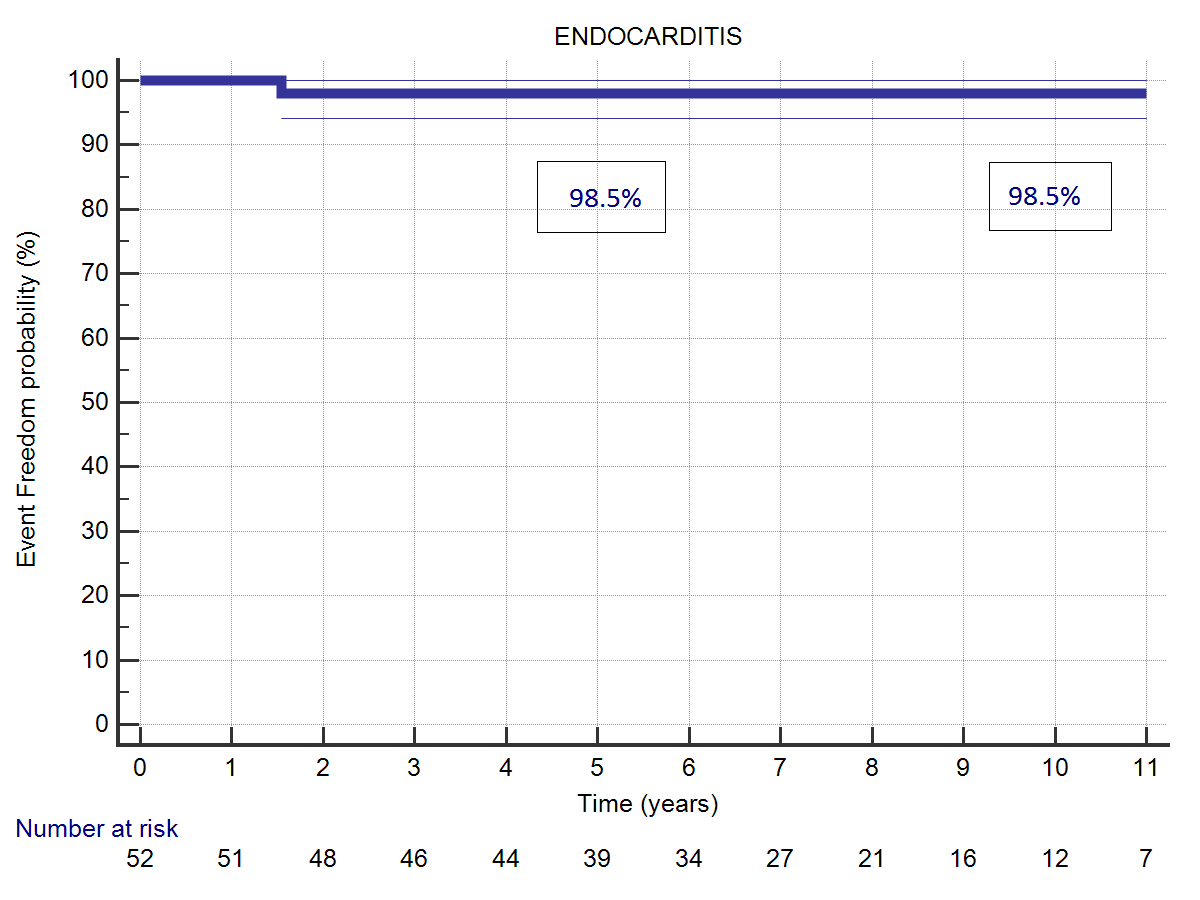
Bacterial endocarditis occurred during follow-up in one case. The reoperation to replace the aortic valve was uneventful. Freedom from bacterial endocarditis at 5 and 11 years was. 98.5% (Figure 3). Incidence of Major Adverse Cardiac and Cerebrovascular Events (MACCE) was. 80.1 % at 5 years, 86.2% at 11 years.
 | 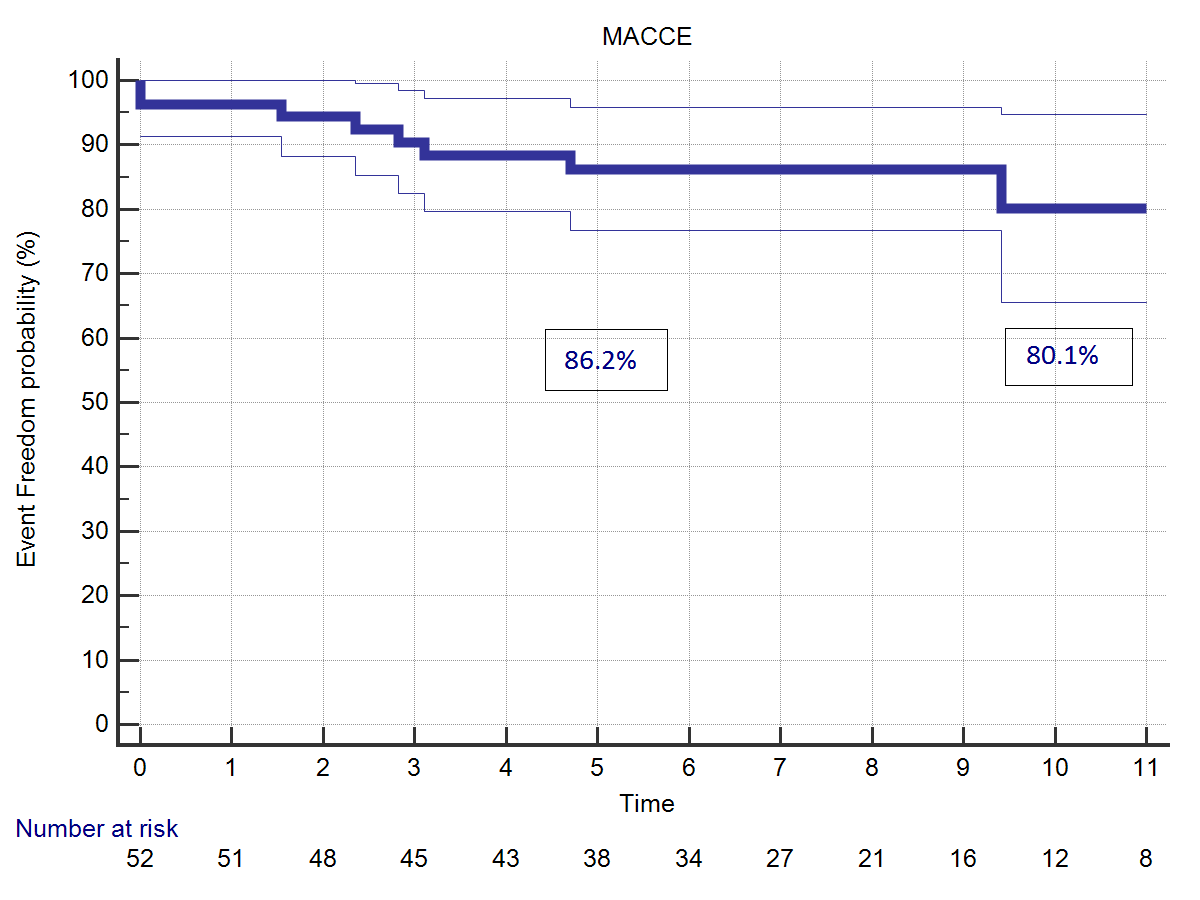 |
| (A) | (B) |
| Figure 3: (A) Freedom from ABE acute bacterial endocarditis. (B) Freedom from MACCE. | |
Aortic valve incompetence of trivial to mild degree at last follow-up was diagnosed by echocardiography in 14 patients (25.9%). Structural valve deterioration was considered in presence of an echo diagnosis of mean aortic valve gradient >20 mmHg or of Aortic Insufficiency (AI) greater than moderate. The four patients with a diagnosis of SVD were in good clinical conditions and remained under close follow-up. At last echo control the degree of valve malfunction was still moderate and did not require its replacement. Freedom from SVD at 5 years was 97.6%, at 11 years follow-up was 90.6% (Figure 4).
Two patients underwent a reoperation during follow-up. It consisted of aortic valve replacement (3.70%), one for acute bacterial endocarditis and one for SVD. This last patient had undergone a Bentall procedure by Shelhigh conduit, and received a successful TAVI implant 10 years later. Freedom from valve-related reoperation was 97.8% at 5 and 11 years follow-up. Transvalvular mean gradient at last follow-up control was 11.70 ± 4.83 mmHg, only slightly increased if compared to hospital discharge evaluation which was 10.88 ± 6.82 mmHg (p=0.001). Likewise, peak gradient had increased from 18.55 ± 5.71 to 20.68 ± 6.32 (p=0.001) (Figure 5). Follow-update are illustrated in Table 2.
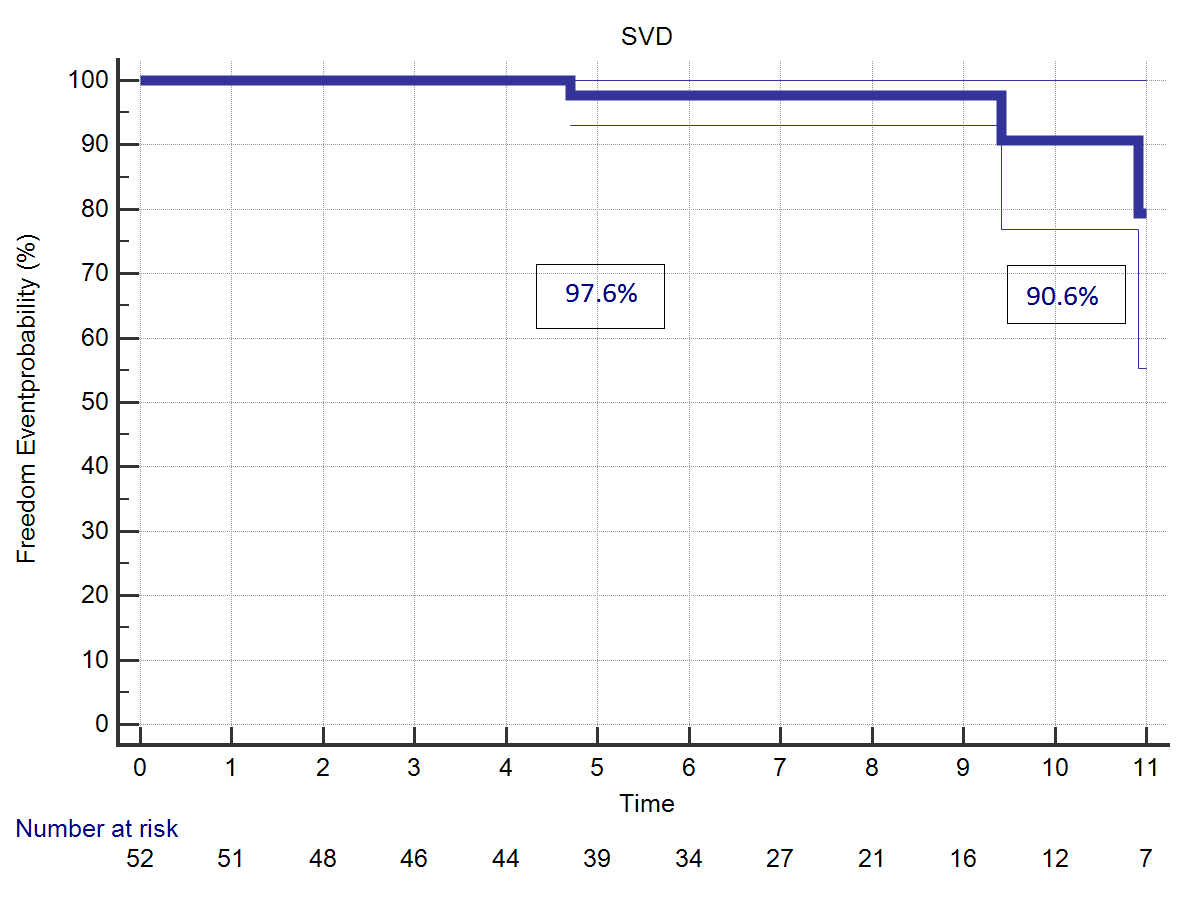 | 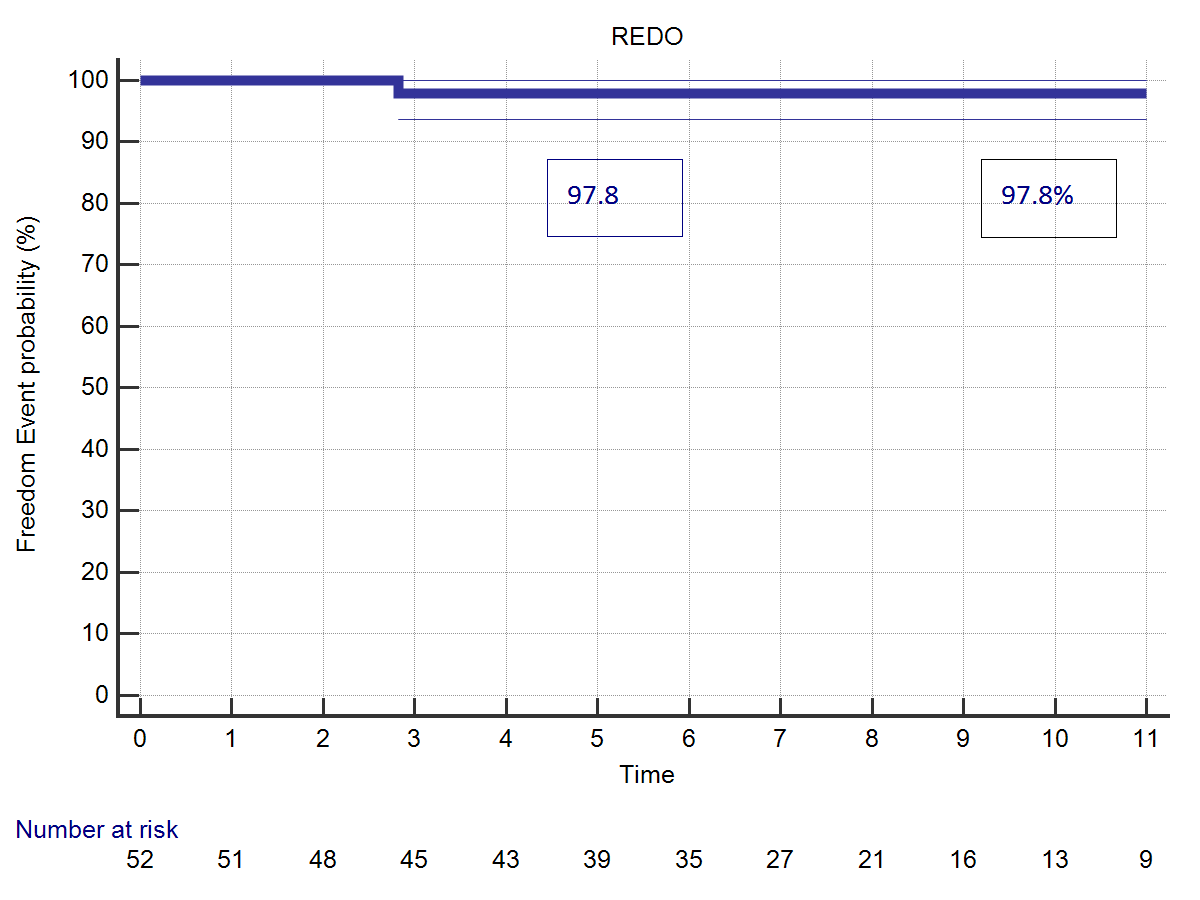 |
| (A) | (B) |
| Figure 4: (A) Freedom from structural valve deterioration. (B) Freedom from valve-related reoperation. | |
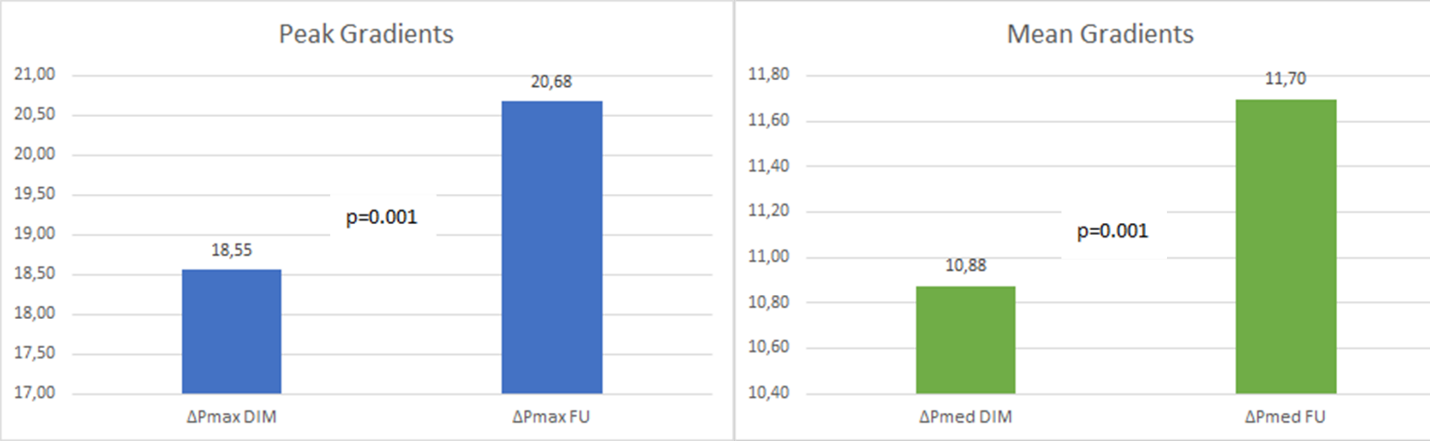
Figure 5: Peak and mean gradients at hospital discharge and at last follow-up.
| BIO-BENTALL | ||
| Mean/n° | St. Dev/% | |
| Overall Deaths (Actual) | 12 | 22.20% |
| Cardiac Deaths (Actual) | 5 | 9.30% |
| NYHA F-up I-II | 49 | 90.71% |
| NYHA F-up III-IV | 5 | 9.29% |
| ΔPmed F-up (mmHg.) | 11.7 | 483.00% |
| AOI (trivial to mild) F-up | 14 | 25.90% |
| ABE (Actual) | 1 | 1.85% |
| SVD (Actual) | 4 | 7.40% |
| Valve Rel. REop (Actual) | 2 | 3.70% |
| Valve Rel Complic. (Actual) | 2 | 3.64% |
| MACCE (Actual) | 10 | 18.50% |
| Abbreviations: NYHA: New York Heart Association; ΔP: left-ventricle-aorta gradient; AOI: aortic insufficiency; ABE: Acute Bacterial Endocarditis; SVD: Structural Valve Deterioration; MACCE: Major Adverse Cardiac and Cerebrovascular Events. | ||
Table 2: follow-up data.
Comments
Bentall operation has represented for many years the gold standard in cases of aortic root and ascending aorta dilatation associated to aortic valve disease. This technique, originally described in 1968 by Bentall De-Bono, was later refined in 1991 by Kouchoukos, who developed the so called ‘button-Bentall modification'. Though originally conceived using a a mechanical valve, the increased demand for biological aortic valve substitutes, more recently led to development and implantation of biological conduits to replace the aortic root. The main reasons for this trend was the avoidance of lifelong anticoagulation, particularly relevant in older patients.
However, also young and active patients affected by aortic root dilatation and aortic valve disease very often ask for a bio-conduit in order to improve their quality of life [3,4], excluding the cases where a valve sparing operation maybe a better option. Indeed, stentless bio-roots may represent a more physiological solution compared to stented prostheses. The avoidance of a valve strut and sewing ring improves the haemodynamic performance and reduces the risk of thromboembolic events [5,6]. Reports of long-term outcomes of bio-roots are comparable to Homograft implantation and to Ross operation [7,8]. In this study we analyzed a cohort of patients affected by aortic root and ascending aorta dilatation with aortic valve disease, who underwent surgical repair by Bentall procedure using only stentless bio-conduits.
We aimed to evaluate the long-term follow-up of patients undergoing stentless bio-Bentall, the overall and cardiac survival, the incidence of MACCE, ABE, SVD and the valve-related reoperations. Finally we intended to assess the prosthesis hemodynamic behavior over time. From Cox regression analysis, age at operation, Logistic EuroSCORE, and patient BSA resulted significant predictors of late overall and cardiac mortality. Additive risk-factors for late cardiac death in all patients resulted : the pre-operative NYHA class, the ABE and the CPB duration. The overall late mortality can be certainly explained by the older age and the impaired clinical status of the patients. From our analysis, hemodynamic performance of the stentless conduits at last follow-up was comparable to that observed at the time of hospital discharge, quite stable over time. While several authors have reported their results after Bentall procedure using composite mechanical or biological stented aortic devices, often compared to valve sparing procedures [9,10], only few papers published in literature analyze the long-term outcomes after Bentall operation performed with only full-stentless bioprosthetic valve conduits [11].
In our opinion, the rationale for using a stentless valve is related to improvement of the hemodynamic performance resulting from larger prosthesis implantation. In our earlier experience we've used the ready-to-implant Shelhigh conduit [12]; subsequently, in the next 30 patients, we began to construct the graft at surgery, by inserting a 3F stentless valve into a straight tube graft, rather than inside a Valsalva graft, as described by Stewart et al [13]. A problem encountered with this type of valve is the occasional finding of false gradients observed at echo evaluation. This evidence is probably due to the peculiar 3F valve design, as reported in a study by Wai Sang Leung et al [14], also confirmed by our observation. And might explain the slightly elevated mean gradient measured by echo in the Bio-Bentall cohort.
Study Limitations
The retrospective design, the limited population and the single surgeon experience are the major limitations of this study. Conversely, the great homogeneity of the patients, receiving a stentless bioprothetic graft in all cases, associated to 100% completeness of follow-up, may reduce the confounding biases often present in similar analysis.
Conclusion
In cases of aortic root dilatation associated to aortic valve disease, biological Bentall procedures using a stentless prosthesis are associated with low early and late cardiac-related mortality and morbidity, acceptable valve durability and reduced rate of valve-related reoperation. This surgical option maybe advisable in young and active patients, when aortic valve sparing operation is not feasible, provided that the indication is correct and the surgical technique very accurate.
Acknowledgment
None.
Conflict of Interest
None.
Funding
The authors hereby declare the absence of any personal or institutional support.
References
01. Bentall H, De Bono A. A technique for complete replacement of the ascending aorta. Thorax. 23(4): 338-9 (1968).
02. Kouchoukos NT, Wareing TH, Murphy SF, Perrillo JB. Sixteen-year experience with aortic root replacement. Results of 172 operations. Ann Surg. 214(3): 308-18 (1991).
03. Yang JA, Neely RC, Stewart AS. Modified bentall procedure with composite biologic grafts. J Card Surg 28(6): 731-735 (2013).
04. Di Bartolomeo R, Botta L, Leone A, Pilato E, Martin-Suarez S, et al. Bio-Valsalva prosthesis: ‘New’ conduit for ‘old’ patients. Interact Cardiovasc Thorac Surg. 7(6): 1062-6 (2008).
05. Urbanski PP. Replacement of the ascending aorta and aortic valve with a valved stentless composite graft. Ann Thorac Surg. 67(5): 1501-2 (1999).
06. Raweh A. Bio-Valsalva or Bio-Integral: Which biological aortic valved conduit has a better hemodynamic performance? J Clin Exp Cardiolog 7: 7 (Suppl) (2016).
07. Christenson JT, Sierra J, Trindade PT, Dominique D, Kalangos A. Bentall procedure using cryopreserved valved aortic homografts: Mid- to long-term results. Tex Heart Inst J. 31(4): 387-91 (2004).
08. De Kerchove L, Rubay J, Pasquet A, Poncelet A, Ovaert C, et al. Ross operation in the adult: Long-term outcomes after root replacement and inclusion techniques. Ann Thorac Surg. 87(1): 95-102 (2009).
09. Leontyev S, Schamberger L, Davierwala PM, Von Aspern K, Etz C, et al. Early and late results after David versus Bentall procedure: A propensity matched analysis. Ann Thorac Surg. 110(1): 120-126 (2020).
10. Gaudino M, Di Franco A, Ohmes LB, Weltert L, Lau C, et al. Biological solutions to aortic root replacement: Valve-sparing versus bioprosthetic conduit. Interact Cardiovasc Thorac Surg. 24(6): 855-861 (2017).
11. Galiñanes M, Meduoyel A, Ferreira I, Sosnowski A. Totally biological composite aortic stentless valved conduit for aortic root replacement: 10-year experience. J Cardiothorac Surg. 6: 86 (2011).
12. Stefanelli G, Pirro F, Macchione A, Bellisario A, Weltert L. Long-term follow-up after Bentall operation using a stentless Shelhigh NR-2000 bio-conduit. J Card Surg. 35(5): 988-995 (2020).
13. Stewart AS, Takayama H, Smith CR. Modified bentall operation with a novel biologic valved conduit. Ann Thorac Surg. 89(3): 938-42 (2010).
14. Sang SLW, Samoukovic G, Buithieu J, DeVarennes B. Falsely elevated valve gradients by echocardiography in the 3f aortic bioprosthesis. Ann Thorac Surg. 96(1): 313-317 (2013).