- Open-Access Publishing
- Quality and Potential Expertise
- Flexible Online Submission
- Affordable Publication Charges
- Expertise Editorial Board Members
- 3 Week Fast-track Peer Review
- Global Visibility of Published Articles
Multiple Sizeable Left Ventricular Thrombi in a Patient with Anti-Phospholipid Syndrome
Eunice Chuah1*, Christopher Alan Brooks2, Ferris Touma1, George Lau1
1Department of Cardiology, Northern Beaches Hospital, New South Wales, Australia
2Department of Neurosurgery, Waikato Hospital, Hamilton, New Zealand
*Corresponding Author: Eunice Chuah, Department of Cardiology, Northern Beaches Hospital, 105 Frenchs Forest Road West, Frenchs Forest, New South Wales 2086, Australia, E-mail: eunicecfp@gmail.com
Received date: December 29, 2021; Accepted date: February 02, 2022; Published date: February 09, 2022
Citation: Chuah E, Brooks CA, Touma F, Lau G. (2022) Multiple Sizeable Left Ventricular Thrombi in a Patient with Anti-Phospholipid Syndrome. J Card Cardi Sur. 1(2):12.
Copyright: © 2022 Chuah E. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Left ventricular thrombus; Anti-phospholipid syndrome; Cardio-embolism; Coagulopathy; Case report
Abstract
Left ventricular thrombus is a morbid complication most often associated with a large territory myocardial infarction. In the present study, we describe a middle-aged male patient presenting acutely with bilateral lower limb ischaemia, secondary to thrombo-emboli and treated with embolectomy. Further investigation revealed precocious coronary artery disease and multiple large left ventricular thrombi, out of proportion to an underlying small region of myocardial infarction. Transient neurological symptoms prompted cerebral magnetic resonance imaging and were determined to be the manifestations of repeated cardio-embolic stroke. Screening for an occult cause of thrombophilia revealed undiagnosed anti-phospholipid syndrome. In this study, we discuss the relevant diagnostic and therapeutic considerations. This case is especially novel in terms of its echocardiography, coronary angiography, and cardiac magnetic resonance imaging. We discuss the choice of anticoagulation as rationalised by the contemporary literature, and the means by which patients are stratified for therapy, in patients with left ventricular thrombus and/or anti-phospholipid syndrome.
Introduction
Left Ventricular Thrombus (LVT) is a complication of Myocardial Infarction (MI) and occurs most often in the setting of apical or large anterior wall MI [1]. Thrombus formation usually occurs within the first three months post-infarct. Virchow’s triad describes concomitant stasis of blood flow, endothelial injury, and coagulopathy as necessary preconditions for thrombosis. Factors which increase the risk and incidence of LVT include advanced age, greater severity ventricular systolic dysfunction, the presence of atrial fibrillation and/or congestive cardiac failure [2]. The incidence of LVT has decreased in recent years due to prompt revascularisation with percutaneous intervention following ischaemic events, resulting in less extensive MI; and the advent of effective anticoagulation agents [1]. Prevention of cardio-embolic complications is essential in reducing the mortality and morbidity associated with MI and LVT.
LVT are often detected on routine post-MI echocardiography, although Cardiac Magnetic Resonance Imaging (CMRI) that demonstrates intraventricular mass T2-weighted signal hypointensity with a concurrent absence of Late Gadolinium Enhancement (LGE), is the gold standard of diagnosis [3]. Furthermore, CMRI can be used to differentiate acute thrombosis from subacute, or chronic and organised lesions, and this is useful in predicting the likelihood of embolisation, and in guiding treatment strategy [4]. Thrombus characteristics of importance include intraventricular location and morphology, with protuberant, mobile, echolucent and large thrombi being more prone to embolisation [1].
The mainstay of treatment of LVT is anticoagulation. Warfarin is the preferred agent in most instances, due to a purportedly lower risk of cardio-embolic stroke or systemic embolism, although this assertion is based primarily on cohort studies. Robinson, et al. [5] detailed a multi-centre retrospective cohort study that audited the medical records of 514 patients treated in North America. Of those anticoagulated, 300 received warfarin and 185 received a Direct-Acting Oral Anticoagulant (DOAC) medication. DOACs were associated with a higher risk of embolisation. Conversely, Bahmaid, et al. [6] detailed a retrospective cohort study of 1631 patients in a Saudi Arabia-based cardiology centre. Of these patients, only eleven were diagnosed with LVT, and seven were treated with a DOAC, while four were treated with warfarin then DOAC. This study suggested a favourable association between DOAC use and LVT resolution. To date, there is no high-quality randomised study addressing this clinical question. There is also no clear consensus regarding the optimal duration of anticoagulation. Some guidelines recommend anticoagulation for a duration of 3-6 months with follow-up imaging thereafter to confirm thrombus resolution [4]. Organised and calcified thrombi may persist and never resolve. The role of surgical thrombectomy remains controversial as the procedure carries high operative risks. It is reserved for patients with mobile, protuberant thrombi resulting in severe left ventricular dysfunction [7].
Case Presentation
A 42-year-old Greek male concreter presented to hospital with bilateral acute lower limb claudication. He reported incidental episodes of transient dysarthria in the three weeks prior. Otherwise, he had no significant past medical history. Acute lower limb ischaemia due to arterial occlusion was suspected. Lower limb and abdominal and pelvic angiography demonstrated complete occlusion of the popliteal arteries bilaterally, and subtotal occlusion of the left common femoral and right common iliac arteries, due to thrombo-emboli. He subsequently underwent embolectomy, with complete resolution of his lower limb symptoms. Investigations to elucidate their source were performed. Transthoracic Echocardiography (TTE) revealed mild segmental apical systolic dysfunction with two sizeable mobile LVT, one attached to the apical septal wall; and one attached to the mid-inferior septal wall, with an additional smaller thrombus attached posteriorly. These were in close apposition to one another. This radiology is depicted in Figure 1. CMRI demonstrated left ventricular wall thinning and hypokinesis, and transmural LGE. These findings were consistent with previous MI and are depicted in Figure 2. The masses demonstrated T2-weighted signal hypointensity and an absence of LGE; findings consistent with LVT. Cerebral magnetic resonance imaging demonstrated bilateral cortical T2 and Fluid-Attenuated Inversion Recovery (FLAIR) hyperintensities with concomitant restricted diffusion. These were thought to be representative of multi-territory cardio-embolic infarcts.
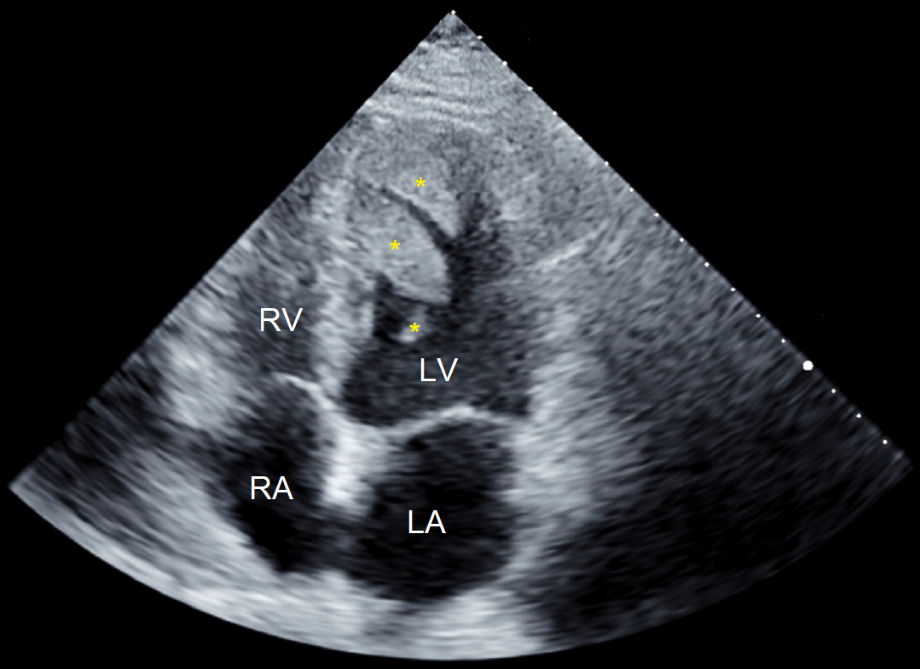
Figure 1: Transthoracic echocardiogram, apical four chamber view. The yellow asterisks distinguish the individual left ventricular thrombi incurred by our patient. Abbreviations: LA: Left Atrium; LV: Left Ventricle; RA: Right Atrium; RV: Right Ventricle.
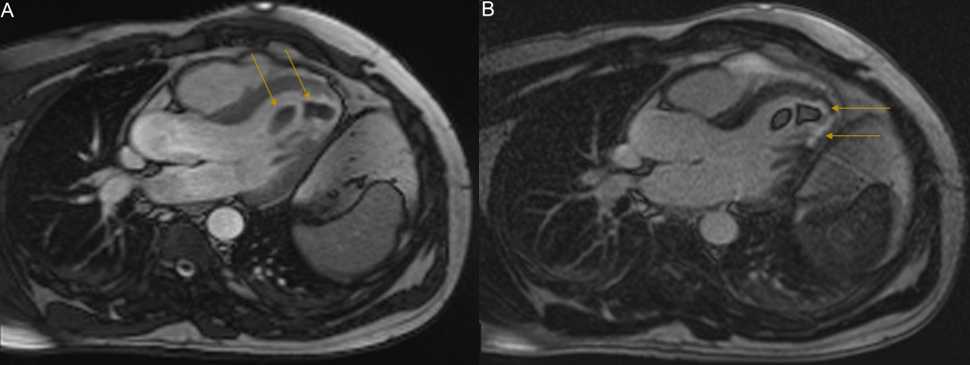
Figure 2: : Cardiac MRI, long axis three-chamber view. (A): Cine sequence image demonstrating two low-signal intensity apical masses, indicated by yellow arrows. Cine imaging demonstrates hypokinesis of the left ventricular apical cap, the apical septum and the apical-inferior segment suggestive of completed myocardial infarction. (B): LGE-MRI sequence. The absence of gadolinium enhancement of the apical masses is characteristic of intraventricular thrombi. Note the enhancement of the thinned apical region consistent with myocardial scarring (yellow arrows). Abbreviations: LGE: Late Gadolinium Enhancement; MRI: Magnetic Resonance Imaging.
Given the unusual clinical presentation; specifically, the size of the LVT, and the absence of significant systolic dysfunction or an associated large territory regional wall motion abnormality; there was suspicion of an underlying coagulopathy. No evidence of malignancy was found on computerised tomography of the chest, abdomen or pelvis. Tumour marker testing was unremarkable. A thrombophilia screen was performed including testing for Anti-Phospholipid Syndrome (APLS). Serum antibody testing demonstrated a high titre (52 Chemiluminescent Units [CU]) of anti-Cardiolipin (aCL) Immunoglobulin M (IgM) and a moderate titre (22 CU) of aCL immunoglobulin G (IgG). There was a medium titre of anti-β2 glycoprotein 1 IgG (23 CU). Testing for the lupus anticoagulant was negative. These findings all but confirmed a diagnosis of APLS.
Subsequent coronary angiography revealed a filling defect due to a non-occlusive thrombus in the left anterior descending artery, and total occlusion of the small obtuse marginal artery. This radiology is depicted in Figure 3. This was managed with anticoagulation given a normal Thrombolysis in Myocardial Infarction (TIMI) flow score of three in the left anterior descending artery, and only mild resulting hypokinesis in a completed infarct of the obtuse marginal artery territory, as espied on TTE and CMRI. Further evaluation with myocardial functional testing was planned for follow-up to reconsider stenting if symptoms of cardiac ischaemia were provokable.
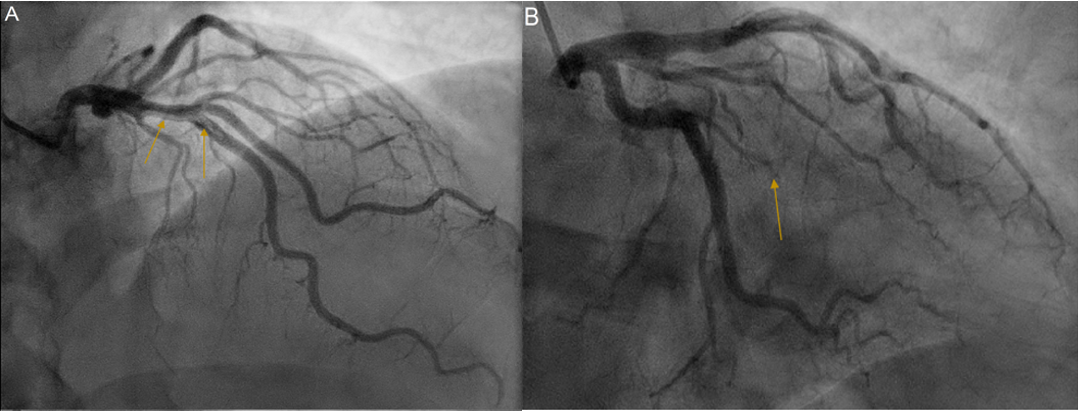
Figure 3: Coronary angiography. (A): RAO 10°/CRA 30° view showing a non-occlusive thrombus of the left anterior descending artery, denoted by the yellow arrows, with TIMI 3 flow. (B): RAO 20°/CAU 20° view showing complete occlusion of the obtuse marginal artery, indicated by the yellow arrow. Abbreviations: CAU: Caudal; CRA: Cranial; RAO: Right Anterior Oblique; TIMI: Thrombolysis in Myocardial Infarction.
Our patient commenced warfarin, with a target International Normalised Ratio (INR) of 2.0-3.0. He remained well for the remainder of his admission. Repeat TTE three months later demonstrated complete resolution of all thrombi. Thereafter, he reported no episodes suggestive of further cerebral or systemic emboli.
Results and Discussion
The revised Sapporo APLS classification criteria (also known as the Sydney criteria) stipulate that a diagnosis of APLS can be established following a clinical event of vascular thrombosis (excluding superficial venous thrombosis) or pregnancy-related morbidity (foetal death or premature birth), with a concomitant demonstration of the serological presence of at least one anti-phospholipid antibody, on two separate tests, taken at least twelve weeks apart [8]. APLS is a complex immunological disorder characterised by recurrent arterial thrombosis, and more commonly, venous thrombosis. It is exceedingly uncommon and has a prevalence of less than 0.05% [9]. There is no known racial predilection [10]. APLS associates with other immunological disorders especially Systemic Lupus Erythematosus [9].
In the treatment of APLS, anticoagulation with warfarin is preferential to that with a DOAC. The existing evidence suggests that warfarin is more effective in prevention of recurrent thrombosis and embolism, especially in those with a history of arterial events [11]. DOAC may be appropriate in patients with warfarin intolerance, compliance issues, and those with lower risk disease, i.e., patients with low or moderate titres of isolated aCL IgM, aCL IgG, or anti-β2 glycoprotein 1 IgG, that require only lower intensity anticoagulation [12]. Several studies have examined the issue of warfarin anticoagulation intensity, and its relationship with APLS disease severity [13]. Arterial thrombosis represents higher risk disease and despite mixed evidence, the contemporary consensus is to warfarinise and aim for an INR greater than 3.0. An alternative therapeutic regimen is to commence low dose aspirin simultaneously with standard intensity warfarinisation, targeting an INR of 2.0-3.0. In all patients, treatment should generally continue in perpetuity [14].
Possible cardiac complications of APLS include: Intracardiac thrombus; valvulopathy including valvular sclerosis and non-infective thrombotic endocarditis; and coronary artery disease due to accelerated atherosclerosis [15]. LVT occurs most often in association with major coronary ischaemic events, with some sources claiming rates as high as 60% in large MI of the anterior wall [1]. Data regarding the incidence of LVT in association with APLS is lacking. Higher titres of anti-phospholipid antibodies are associated with arterial and intracardiac thrombi with shorter times between thrombosis recurrence [16], suggesting that cardiac events represent higher risk disease. There is some speculation that LVT has a protective role following acute MI, specifically in that it provides mechanical support and prevents left ventricular myocardial rupture [2], although this process is likely exaggerated in the context of a prothrombotic coagulopathy like APLS.
In the present study, we propose that a clinically silent MI involving occlusion of the obtuse marginal artery occurred, potentiating a small region of the endocardium for thrombosis, and the prothrombotic milieu of underlying serum anti-phospholipid antibodies, caused the formation of large and multiple LVT. Our patient subsequently presented with the symptoms of subacute cerebral and acute systemic thrombo-embolism. An alternative hypothesis is that LVT preceded the obtuse marginal artery lesion, and that its occlusion was also the result of thromboembolism. This latter theory has the disadvantage of not explaining the aetiology of the LVT and is thus less likely.
Conclusion
Echocardiography and CMRI are the modalities of choice in the diagnosis of LVT. LVT associated with a small territory MI is a rare occurrence. In these patients, coagulopathy must be considered. APLS is a condition characterised by a diathesis for thrombosis and is associated with premature coronary artery disease. Contemporary evidence suggests long-term warfarinisation is the best therapy in terms of prevention of recurrent thrombotic events in patients with APLS, and prevention of cardiac emboli in patients with LVT. Further research is required to elucidate the role of DOAC medications and variant intensity anticoagulation regimens in both conditions.
References
01. Hudec S, Hutyra M, Precek J, Latal J, Nykl R, et al. Acute myocardial infarction, intraventricular thrombus and risk of systemic embolism. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 164(1): 34-42 (2020).
02. Delewi R, Zijlstra F, Piek JJ. Left ventricular thrombus formation after acute myocardial infarction. Heart. 98(23): 1743-9 (2012).
03. Paydarfar D, Krieger D, Dib N, Blair RH, Pastore JO, et al. In vivo magnetic resonance imaging and surgical histopathology of intracardiac masses: Distinct features of subacute thrombi. Cardiology. 95(1): 40-7 (2001).
04. Roifman I, Connelly KA, Wright GA, Wijeysundera HC. Echocardiography vs. cardiac magnetic resonance imaging for the diagnosis of left ventricular thrombus: A systematic review. Can J Cardiol. 31(6): 785-91 (2015).
05. Robinson AA, Trankle CR, Eubanks G, Schumann C, Thompson P, et al. Off-label use of direct oral anticoagulants compared with warfarin for left ventricular thrombi. JAMA Cardiol. 5(6): 685-92 (2020).
06. Bahmaid RA, Ammar S, Al-Subaie S, Soofi MA, Mhish H, et al. Efficacy of direct oral anticoagulants on the resolution of left ventricular thrombus-A case series and literature review. JRSM Cardiovasc Dis. 8: 2048004019839548 (2019).
07. Cruz Rodriguez JB, Okajima K, Greenberg BH. Management of left ventricular thrombus: A narrative review. Ann Transl Med. 9(6): 520 (2021).
08. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, et al. International consensus statement on an update of the classification criteria for definite Antiphospholipid Syndrome (APS). J Thromb Haemost. 4(2): 295-306 (2006).
09. Duarte-García A, Pham MM, Crowson CS, Amin S, Moder KG, et al. The epidemiology of antiphospholipid syndrome: A population-based study. Arthritis Rheumatol. 71(9): 1545-52 (2019).
10. Uthman I, Khamashta M. Ethnic and geographical variation in antiphospholipid (Hughes) syndrome. Ann Rheum Dis. 64(12): 1671-6 (2005).
11. Cohen H, Hunt BJ, Efthymiou M, Arachchillage DR, Mackie IJ, et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): A randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol. 3(9): e426-36 (2016).
12. Rodziewicz M, D'Cruz DP. An update on the management of antiphospholipid syndrome. Ther Adv Musculoskelet Dis. 12: 1759720x20910855 (2020).
13. Ghembaza A, Saadoun D. Management of antiphospholipid syndrome. Biomedicines. 8(11): (2020).
14. Kolitz T, Shiber S, Sharabi I, Winder A, Zandman-Goddard G. Cardiac manifestations of antiphospholipid syndrome with focus on its primary form. Front Immunol. 10: 941 (2019).
15. Mavrogeni SI, Sfikakis PP, Kitas GD, Kolovou G, Tektonidou MG. Cardiac involvement in antiphospholipid syndrome: The diagnostic role of non-invasive cardiac imaging. Semin Arthritis Rheum. 45(5): 611-6 (2016).
16. Forastiero R. Multiple antiphospholipid antibodies positivity and antiphospholipid syndrome criteria re-evaluation. Lupus. 23(12): 1252-4 (2014).