- Open-Access Publishing
- Quality and Potential Expertise
- Flexible Online Submission
- Affordable Publication Charges
- Expertise Editorial Board Members
- 3 Week Fast-track Peer Review
- Global Visibility of Published Articles
QT Interval Changes in Patients Receiving SARS-COV2 Treatments
Saeide Bahrani1,4, Mehrzad Salmasi2, Masoumeh Sadeghi3, Fatemeh Nouri5, Hamidreza Roohafza6, Forogh Soltaninejad7, Zahra Teimouri-jervekani3*
1Student Research Committee, Isfahan University of Medical Sciences, Isfahan, Iran
2Department of Internal Medicine, School of Medicine, Isfahan University of medical Sciences, Isfahan, Iran
3Cardiac Rehabilitation Research Center, Cardiovascular Research Institute, Isfahan University of Medical Sciences, Isfahan, Iran
4Hypertension Research Center, Cardiovascular Research Institute, Isfahan University of Medical Sciences, Isfahan, Iran
5Isfahan Cardiovascular Research Center, Cardiovascular Research Institute, Isfahan University of Medical Sciences, Isfahan, Iran
6Psychosomatic Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
7Department of Medicine, Pulmonary Ward, Isfahan University of Medical Sciences, Isfahan, Iran
*Corresponding Author: Zahra Teimouri-jervekani, Cardiac Rehabilitation Research Center, Cardiovascular Research Institute, Isfahan University of Medical Sciences, Isfahan, Iran; E-mail: Zahra.teimouri@med.mui.ac.ir
Received date: June 11, 2021; Accepted date: July 09, 2021; Published date: July 16, 2021
Citation: Bahrani S, Salmasi M, Sadeghi M, Nouri F, Roohafza H, et al. (2021) QT Interval Changes in Patients Receiving SARS-COV2 Treatments. J Card Cardi Sur. 1:04.
Copyright: © 2021 Bahrani S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Electrocardiography, Hydroxychloroquine, QTc interval, SARS-CoV2
Abstract
Background: Definite treatment for the COVID-19 is not approved. The advantages and disadvantages of treatment options have to be considered in all patients.
Aims: In this study we aimed to determine corrected QT (QTc) interval changes based on the treatment options which can potentially prolong QT interval and possible consequences.
Methods: 256 admitted patients with the diagnosis of SARS-CoV2 infection enrolled. The QTc interval, at the time of admission and maximal value during hospital stay was evaluated. ΔQT for calculated. QTc was compared between critically ill patients and patients discharged in different groups of treatment including no drug, Hydroxychloroquine, Azithromycin, Lopinavir/Ritonavir (kaletra), and Oseltamavir.
Results: The mean age was 59.4 (15.68) years. Approximately 40 % of increases in QT interval was less than 40 ms. ΔQT increased significantly among critically ill patients during hospital stay and it was also significantly higher at the baseline in these patients (p value: 0.014). After adjustment of patients for confounding variables ANOVA test revealed no significant difference between different drug groups (p value=0.857). There was no significant correlation between HCQ dosage and ΔQT (p value=0.656).
Conclusion: QTc interval increases significantly among critically ill patients during hospital stay. Short-term therapy of alone or in combination therapy of Hydroxychloroquine with other potentially QTc-prolonging drugs for treatment of patients with COVID-19 infection seems to be safe. Other comorbidities and severity of disease may be the main risk factors of QTc prolongation.
Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV2) pandemic affect millions of people worldwide from April 2020, but there are no approved treatments to date [1]. This pandemic makes therapeutic and prognostic challenges worldwide. Patients with comorbidities especially cardiovascular diseases are at the highest risk of involvement and worse outcomes [2]. Besides, therapeutic strategies and drugs for treatment could potentially affect the cardiovascular system, especially the conduction system and consequently, cause cardiac morbidity and even mortality. One of the most combinations for treatment of COVID-19 pneumonia was Hydroxychloroqiune/Azithromycin that leads to corrected QT (QTc) interval prolongation and life-threatening arrhythmias including mainly Torsade des pointes consequently according to previous reports [3-5]. Other treatment options are also have been used including kaletra (Lopinavir-Ritonavir) [6], Oseltamavir [7], and the combination of these drugs. All of these drugs mentioned before have the reported potency to affect QT interval in patients especially with underlying disease [8,9], but the effect of the combination of these drugs with hydrxychloquin remained a question. As the definite treatment for the COVID-19 is not recognized, the advantage and this advantage of treatment options have to be considered in all patients. Thus in this study we aim to determine QTc interval changes base on the treatment among patients with SARS-CoV2.
Methods
Patient’s setting
All patients admitted to Khorshid Hospital, Isfahan, Iran from January to April 2020 with the definite diagnosis of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV2) by Real-Time Polymerase Chain Reaction (Rt-PCR) of the nasopharyngeal and oropharyngeal specimens were enrolled. The ethical committee of Isfahan University of Medical Sciences, Isfahan, Iran approved this study.
Measurements and outcomes
Demographic characteristics of patients and the risk factors including age, gender, comorbidities, and history of smoking or opium addiction recruited. Electrocardiography was also performed in all patients. QTc interval (corrected QT interval) in all patients were measured. QT interval corrected by the following formula: QTc=QT+1.75 (HR-60)
QTc interval compared between patients with critical clinical condition (patients who expired during hospitalization and patients with ICU care) and patients who discharged without any ICU care. According to drugs that may affect the QTc interval, groups of treatment categorized as Hydroxychloroquine+Azithromycin, Hydroxychloroquine+Levofloxacin+Oseltamavir, Hydroxychloroquine+Kaletra+Azithromycin, Hydroxychloroquine+Kaletra+Levofloxacin+Oseltamavir, and no drug for treatment. QTc interval of all patients at the time of admission evaluated. It was also evaluated further in patients who had more ECG evaluation in the days of hospitalization.
Statistical analysis
Continuous variables were described with mean and standard deviation. Variables were evaluated for normality of distribution and log transform was used if a variable was considered non-normally distributed. Frequency was also used to describe descriptive statistics. For the analysis of quantitative parameters, Exact Fisher's exact test, and for the descriptive parameters, Chi-Square test was used. The significance level was set 0.05. SPSS version 25 (IBM, Armonk, NY) were used for all the analyses.
Results
Two hundred and fifty-six patients with the diagnosis of COVID-19 were enrolled in the study. Demographic characteristics of all patients and drug history of them before admission are shown in Table 1. Patients had the mean age of 59.4 (15.68) years. 19.9% (51) of them had the prior history of IHD.
| Baseline characteristics | |
| Age (mean(S.D)) | 59.4 (15.68) |
| Male, n (%) | 164 (64.1%) |
| Diabetes mellitus, n (%) | 73 (28.5%) |
| Hypertension, n (%) | 96 (37.5%) |
| History of Ischemic Heart Disease, n (%) | 51 (19.9%) |
| Drug history | |
| (a) Anti-platelet (ASA/Plavix) | 9 (3.5%) |
| (b) Statin | 46 (18%) |
| (c) Digoxin | 5(2%) |
| (d) Oral Anticoagulants (warfarin/NOAC) | 11 (4.3%) |
| (e) Nitrates | |
| (f) Beta blocker | 12 (4.7%) |
| (g) ACEI/ARB | 49 (19.1%) |
| (h) Calcium Channel Blocker | 73 (28.5) |
| (i) Diuretics (Frusemide/Thiazide) | 23 (9%) |
| (j) Aldosterone antagonists | 31 (12.1%) |
| (k) Benzodiazepine | 4 (1.6%) |
| (l) Sodium valproate | 5 (2%) |
| (m) Quetiapine | 3 (1.2%) |
| (n) Levothyroxine | 3 (1.2%) |
| (o) Corticosteroid | 17 (6.6%) |
| (p) SSRI | 11 (4.3%) |
| (q) TCA | 7 (2.7%) |
| (r) Immunosuppressive | 7 (2.7%) |
| Smoker, n (%) | 12 (4.7%) |
| Addiction, n (%) | 11 (4.3%) |
| Abbreviations: ACEI: Angiotensin Converting Enzyme Inhibitor; ARB: Angiotensin Receptor Blocker; SSRI: Selective Serotonin Reuptake Inhibitors; TCA: Tricyclic Antidepressants; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; CRP: C-Reactive Protein; BUN: Blood Urea Nitrogen | |
Table 1: Baseline characteristics of patients with SARS-CoV2 (n=256).
QTc interval compared between patients with critical clinical condition (patients who expired during hospitalization and patients with ICU care) and patients who discharged without any ICU care using non-parametric Mann-Whitney U test due to not having normal distribution, in Table2. The prolongation of the QTc interval in critically clinical condition patients from a baseline average of 412.75 (41.4) ms (mean (S.D)) to a maximal average value of 446.89 (54.78) ms was seen. At the beginning, the mean (SD) of QTc interval in both group showed no significant differences (p value=0.235), but during hospital stay, the mean (SD) of QTc interval in patients with critical clinical condition, increased significantly (p value: 0.001). ΔQT which was calculated by the differences of QTc1 and QTc2, also increased significantly among patients with critical clinical condition (p value: 0.014).
| Critically ill patients | Discharge without ICU care | t value | P value | |
| QTc1 (n=253) | 412.75 (41.40) | 406.04 (29.98) | 1.195 | 0.235 |
| QTc2 (n=137) | 446.89 (54.78) | 417.20 (33.58) | 3.511 | 0.001 |
| ΔQT (n=137) | 29.89 (63.62) | 5.52 (34.29) | 2.532 | 0.014 |
| Abbreviation: QTc: Corrected QT interval | ||||
Table 2: Comparison the QTc interval between patients stratified by critical clinical condition (ICU care and expired) and discharged without ICU care (n=256).
To evaluate the effect of drugs on the QTc interval, we categorized patients in Hydroxychloroquine+Azithromycin, Hydroxychloroquine+Levofloxacin+Oseltamavir, Hydroxychloroquine+Kaletra+Azithromycin, Hydroxychloroquine+Kaletra+Levofloxacin+Oseltamavir, and no drug for treatment group. ΔQT compared between groups, using ANOVA test (Table 3). ANOVA test revealed no significant difference between different drug groups (p value=0.857).
| ANOVA | |||||
| Sum of Square | df | Mean Square | F | Sig. | |
| Between Groups | 2411.49 | 4 | 602.872 | 0.33 | 0.857 |
| Within groups | 177313.984 | 97 | 1827.979 | ||
| Total | 179725.474 | 101 | |||
Table 3: ANOVA test between different groups of treatment for evaluation of ΔQT.
After adjustment of patients for confounding variables including age, gender and prior history of TCA consumption, analysis of covariance (ANCOVA) showed no significant difference between different drug groups (Table 4 and Figure 1).
| Group of treatment | Mean | Std. Deviation | N | Subset of α=0.05 |
| No drugs | 21.41 | 61.76 | 16 | 21.41 |
| Hydroxychloroquine+Azithromycin | 6.58 | 34.06 | 16 | 6.58 |
| Hydroxychloroquine+Kaletra+Azithromycin | 13.07 | 43.16 | 22 | 13.07 |
| Hydroxychloroquine+Levofloxacin+Oseltamavir | 7.88 | 37.70 | 29 | 7.88 |
| Hydroxychloroquine+Kaletra+Levofloxacin+Oseltamavir | 12.76 | 36.35 | 19 | 12.76 |
| Total | 11.83 | 42.18 | 102 |
Table 4: Mean ± SD of ΔQT in different groups of treatment.
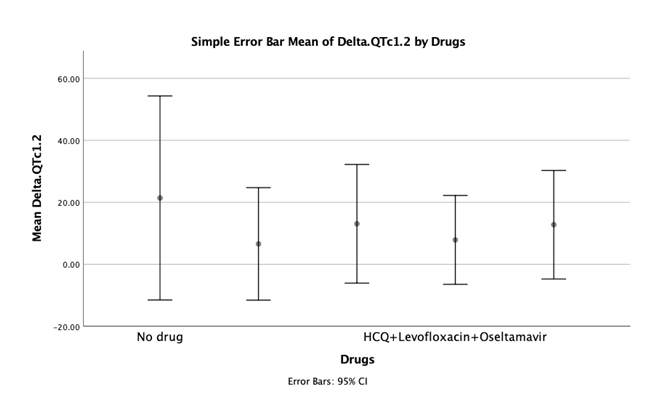
Figure 1: Simple error bar for the Mean of ΔQT in different drugs.
The correlation between Hydroxychloroquine dosage and ΔQT evaluated based on pearson correlation. No significant correlation between HCQ dose and ΔQT was showed (p value=0.656). ΔQT categorized as 0-20, 20-40, 40-60, and >60 ms (Figure 2). 27.9% of patients had the ΔQT changes in average of 0-20 ms. 4 patients have QTc interval more than 500 ms during hospital stay and three patients had QTc interval more than 500 ms at the admission. One patient died from sustained ventricular tachycardia, without evidence of electrolyte abnormalities and without severe QTc prolongation.
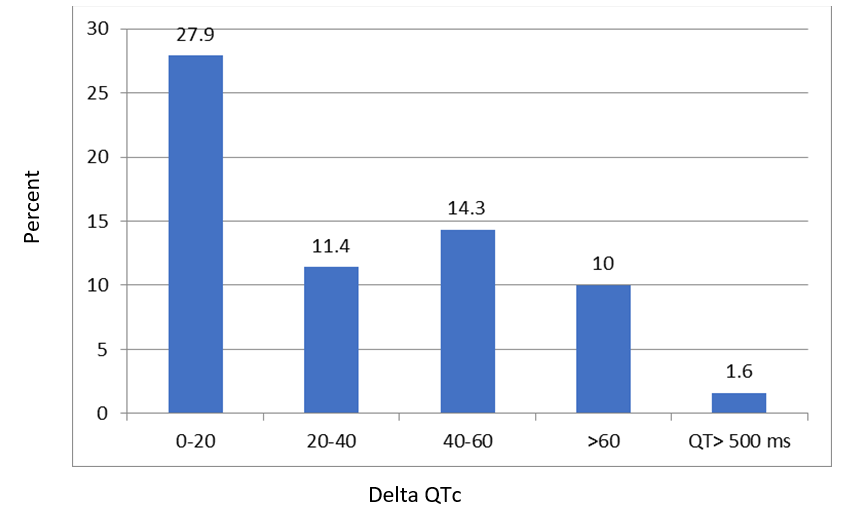
Figure 2: ΔQT categorization among all patients.
Discussion
According to our findings, QTc interval increases significantly among patients with critically clinical condition during hospital stay. The mean of QTc interval at the baseline in the patients with critically clinical condition was higher than others, although this difference was not statistically significant. Critically ill patients not only had higher QTc interval, but also experienced more increase in QTc during hospital stay. QTc increase among patients who received different treatment regimen including Hydroxychloroquine and other agents which potentially increase QTc, were mild. QTc changes among patients who received different groups of treatment revealed no significant differences. Indeed, no specific drug would change the electrophysiological characteristics of these patients.
Healthcare professionals attempted to find immediate treatment options to face this pandemic challenges which leads to several experimental treatments with intermediate clinical evidence [10,11]. HCQ was used as first-line regiment after that some clinical reports provided its clinical advantages [12]. Prescribing Hydroxychloroquine alone in the short-term treatment of patients with COVID-19 regardless of clinical setting can prolong QTc moderately which was irrelevant to life-threatening arrhythmias and sudden cardiac death. Of course other clinical conditions including fever can affect these results [13]. In addition, the effect of Hydroxychloroquine/Azithromycin was shown in small studies [14] which lead many clinicians to evaluate and prescribe many therapeutic combinations. In younger patients who were otherwise healthy, the combination of Hydroxychloroquine/Azithromycin led to just a mild QTc prolongation [15] but in another study, this combination leaded to a significant QTc prolongation [3]. These differences in line with our findings may be due to severity of disease and other comorbidities [16]. Another case report of patient with COVID-19 with severe QTc prolongation who had significant troponin rise, revealed the QTc prolongation may be due to other mechanisms other than drug toxicity, for instance myocardial inflammation [17]. All of our four patients who had QTc more than 500 ms, had the near normal QTc at the admission and all of them died during hospital stay. These findings are in line with other studies supported the importance of clinical condition and comorbidities in QTc prolongation. None of these patients who are at high risk of torsade des pointes due to severely prolonged QTc and life-threatening arrhythmia, experienced cardiac arrhythmia during continuous heart monitoring in Intensive Care Unit.
The first limitation of our study is the lack of long ad continuous heart monitoring and thus, the arrhythmic outcome assessment was not fully completed. In addition, the HCQ dose used in clinical practice varied across patients.
Conclusion
In conclusion QTc interval increases significantly among patients with clinically critical condition during hospital stay. Short-term therapy of alone or in combination therapy of Hydroxychloroquine with other potentially QTc-prolonging drugs for treatment of patients with COVID-19 infection seems to be safe. Other comorbidities and severity of disease may be the main risk factors of QTc prolongation.
Ethical Statement
The study obtained the ethical approval the Research Ethics Committee of Isfahan University of Medical Sciences with approval ID: IR.MUI.RESEARCH.REC.1299.002.
Conflict of Interests
Authors declare there are no conflicts of interest.
Funding
This study was supported by Isfahan University of Medical Science [Grant number, 198326].
References
1. Giwa AL, Desai A. Novel coronavirus COVID-19: An overview for emergency clinicians. Emerg Med Pr. 22(S2):1-21 (2020).
2. Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in COVID-19. N Engl J Med. 382: e102 (2020).
3. Chorin E, Dai M, Shulman E, Wadhwani L, Bar-Cohen R, et al. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin. Nat Med. 26(6): 808-809 (2020).
4. Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. 5(9): 1036-1041 (2020).
5. Chorin E, Wadhwani L, Magnani S, Dai M, Shulman E, et al. QT interval prolongation and torsade De pointes in patients with COVID-19 treated with Hydroxychloroquine/azithromycin. Hear Rhythm. 17(9): 1425-1433 (2020).
6. Lim J, Jeon S, Shin H-Y, Kim MJ, Seong YM, et al. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: The application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Korean Med Sci. 35(6): e79 (2020).
7. Muralidharan N, Sakthivel R, Velmurugan D, Gromiha MM. Computational studies of drug repurposing and synergism of lopinavir, oseltamivir and ritonavir binding with SARS-CoV-2 Protease against COVID-19. J Biomol Struct Dyn. 39(7) 2673-2678 (2020).
8. Dutkowski R, Thakrar B, Froehlich E, Suter P, Oo C, et al. Safety and pharmacology of oseltamivir in clinical use. Drug Saf. 26(11):787-801 (2003).
9. Hunt K, Hughes CA, Hills-Nieminen C. Protease inhibitor-associated qt interval prolongation. Ann Pharmacother. 45(12): 1544-50 (2011).
10. Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 14(1): 72-73 (2020).
11. Cao B, Wang Y, Wen D, Liu W, Wang J, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 382: 1787-1799 (2020).
12. Esposito S, Tagliabue C, Bosis S, Principi N. Levofloxacin for the treatment of Mycoplasma pneumoniae-associated meningoencephalitis in childhood. Int J Antimicrob Agents. 37(5): 472-5 (2011).
13. Gasperetti A, Biffi M, Duru F, Schiavone M, Ziacchi M, et al. Arrhythmic safety of hydroxychloroquine in COVID-19 patients from different clinical settings. Europace. 22(12): 1855-1863 (2020).
14. Wang M, Cao R, Zhang L, Yang X, Liu J, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 30(3):269-71 (2020).
15. Hancox JC, Hasnain M, Vieweg WVR, Crouse ELB, Baranchuk A. Azithromycin, cardiovascular risks, QTc interval prolongation, torsade de pointes, and regulatory issues: a narrative review based on the study of case reports. Ther Adv Infect Dis. 1(5):155-65 (2013).
16. Fernandes FM, Silva EP, Martins RR, Oliveira AG. QTc interval prolongation in critically ill patients: Prevalence, risk factors and associated medications. PLoS One. 13(6): e0199028 (2018).
17. Merino JL, Martinez-Cossiani M, Iniesta A, Escobar C, Rey JR, et al. COVID-19 and QT interval prolongation: More than just drug toxicity? EP Eur. 22(10): 1479 (2020).