- Open-Access Publishing
- Quality and Potential Expertise
- Flexible Online Submission
- Affordable Publication Charges
- Expertise Editorial Board Members
- 3 Week Fast-track Peer Review
- Global Visibility of Published Articles
Spontaneous Coronary Artery Dissection: An Overview of Pathophysiology, Diagnosis, and Management
Usman Sarwar1, Nikky Bardia1, Hassan Tahir2*, Ali Hussain3, Amod Amritphale1
1Department of Cardiology, University of South Alabama, Mobile, AL, USA
2Department of Cardiology, Heart Lung Vascular Institute, University of Tennessee Medical Center, Knoxville, TN, USA
3Department of Cardiology, Sentara Albemarle Medical Center, Elizabeth City, NC, USA
*Corresponding Author: Hassan Tahir, Department of Cardiology, Heart Lung Vascular Institute, University of Tennessee Medical Center, Knoxville, TN, USA, E-mail: doctorhassantahir@gmail.com
Received date: August 02, 2021; Accepted date: August 25, 2021; Published date: September 01, 2021
Citation: Sarwar U, Bardia N, Tahir H, Amritphale A. (2021) Spontaneous Coronary Artery Dissection: An Overview of Pathophysiology, Diagnosis, and Management. J Card Cardi Sur. 1(2):11.
Copyright: © 2021 Tahir H. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Spontaneous coronary artery dissection; Acute coronary syndrome; Intravascular ultrasound; Optical coherence tomography
Abstract
Spontaneous Coronary Artery Dissection (SCAD) is non-atherosclerotic disease that can lead to luminal obstruction. The underlying pathophysiological mechanism is the separation of coronary artery intima from media or media from adventitia, resulting in hematoma that can cause longitudinal extension or axial compression of vessel resulting in occlusion of the true lumen of coronary artery. New imaging techniques, such as intravascular ultrasound (IVUS) and Optical Coherence Tomography (OCT), have led to improved diagnosis. The management depends upon several factors, including presenting clinical scenario, hemodynamically instability, and coronary anatomy features.
Introduction
Spontaneous Coronary Artery Dissection (SCAD) is a particularly important cause of the acute coronary syndrome, explicitly in the younger women population. Although the first case was diagnosed as early as 1931 [1], due to lack of good imaging technologies, this fatal disease remains under-reported, now with new imaging techniques, i.e., intravascular ultrasound (IVUS) and Optical Coherence Tomography (OCT), the reported prevalence of this disease has been increased significantly.
Definition
SCAD is defined as a separation of coronary artery intima from media or media from adventitia resulting in hematoma that can cause longitudinal extension or axial compression of vessel resulting in occlusion of the true lumen of coronary artery. Coronary artery dissection due to trauma or iatrogenic causes does not come under SCAD.
Literature Review
Epidemiology
According to a position paper on SCAD by Adlam review of different SCAD studies, the median age of affected patients was between 44 to 53 years, with more than 90% population consisting of women [2]. The presence of traditional risk factors of atherosclerotic disease, i.e., hypertension, hyperlipidemia, and smoking, is usually not frequently present in SCAD. In acute myocardial infarction, SCAD is found in 4% of the cases. Still, interestingly, it is attributed to 40% of the cases in peripartum or early postpartum patients presenting with acute myocardial infarction [3,4].
Pathophysiology
SCAD is a non-atherosclerotic disease. The final common pathway is luminal obstruction by intramural hematoma, as described earlier. The exact etiology of this intramural hemorrhage or hematoma formation is unknown, but there are a couple of proposed mechanisms. It can be due to the tear of an intimal layer, or it can also form by bleeding of small blood vessels known as vasa vasorum [5]. As a result, the hematoma that is formed obstructs the coronary blood flow and causes myocardial infarction or ischemia. Histological analysis of involved arteries did show eosinophilic infiltrates that suggest maybe there is a periarteritis component, but it was not determined as cause and thought to be injury-related changes [6] (Figure 1).
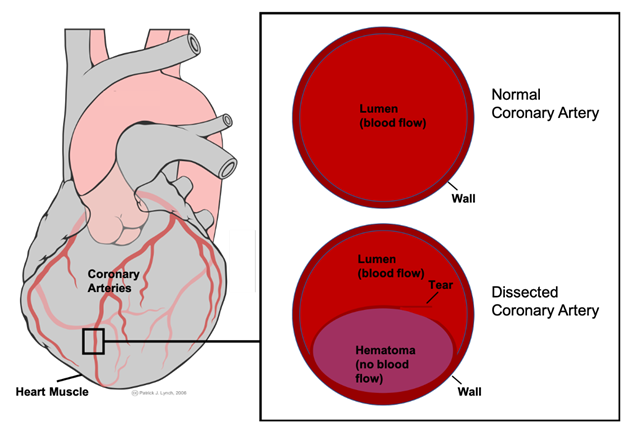
Figure 1: Mechanism of lumen obstruction in spontaneous coronary artery dissection.
Clinical Presentation and Manifestations
In patients with SCAD, the most common presenting complaint is chest pain, other symptoms that have also been reported but are less prominent include back pain, neck pain, diaphoresis, and dyspnea [7]. According to one study, most patients had ACS (Acute Coronary Syndrome) on initial presentation. ST-elevation MI (Myocardial Infarction) was found in more than 25% of patients [8]. Cardiogenic shock is usually presented in 2 to 19% of the patients, and ventricular arrhythmias were present in 3-4% of the patients [9-11]. Regarding triggers, emotional stress, especially in women, was one of the significant preceding events and was present in 50% of the cases. On the other hand, in men, intense exertional exercise was found as a more common trigger. Interestingly, patients who were diagnosed with acute myocardial infarction reported to have similar chest pain in the past, for which most probably due to their younger age, they did not seek any medical attention, so it is common in patients with SCAD to have old infarct on initial imaging without any known history or risk factors for coronary artery disease [12].
Types and classification
There are three main categories of SCAD according to anatomical and angiographic classification.
Type I SCAD has the more typical appearance of arterial dissection on angiography and presents as multiple lumens radiolucent due to intimal tear. This type of SCAD is easier to recognize than other types but is present in only up to 25% of SCAD cases [13].
Type 2 SCAD is the most common and has been seen in 60 to 75% of patients. In this type of SCAD, there is an absence of intimal tear and diffuse intramural hematoma present that cause stenosis of a long segment of the affected vessel. In most cases, it’s usually Left Anterior Ascending (LAD) that is affected. On coronary angiogram, it appears as a diffusely narrowed long segment with a sudden change in caliber/diameter of the vessel.
Type 3 SCAD is also difficult to diagnose because of its resemblance to atherosclerotic disease. It can present as 100% focal stenosis or total occlusion, to diagnose this type of advanced coronary imaging is often required.
The most common artery involved is distal LAD followed by obtuse marginal. SCAD can be multivessel in 14 % of cases, according to one study [14] (Figure 2).
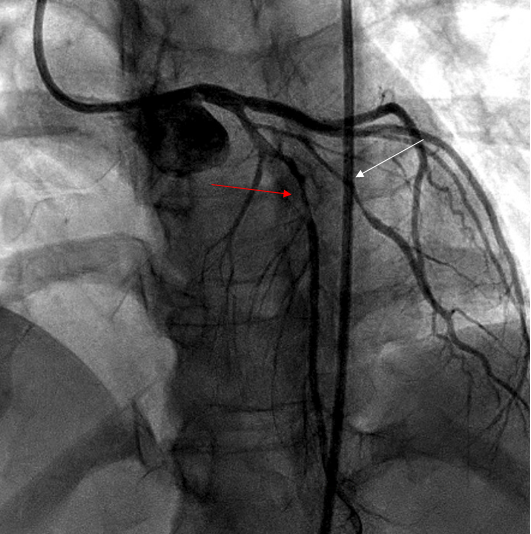
Figure 2: Coronary angiogram showing type 2 SCAD in LAD (red arrow) and diagonal (white arrow) in 35-year-old women presented with chest pain 3 weeks postpartum.
Diagnosis of spontaneous coronary artery dissection
Diagnosis of SCAD is challenging because many other diseases like coronary artery vasospasm or coronary thromboembolism can mimic SCAD. A brief review of imaging techniques for the diagnosis of SCAD is given below.
Role of multimodality imaging in diagnosis
Coronary angiography is the mainstay of diagnosis, especially in active chest pain, for spontaneous coronary artery dissection. If a diagnosis is unclear new advanced imaging modalities can be used like IVUS or OCT. OCT is especially beneficial due to its remarkably high spatial resolution of 15 um, but invasive imaging modalities carry the risk of dissection flap propagation; therefore, it should be done when the diagnosis is unclear or difficult to make, like in type III SCAD. If intervention is being performed, imaging is more helpful to prevent stenting in the false lumen [15].
Coronary Computed Tomography Angiography (CCTA) is also a useful modality with its limitation. It can detect spontaneous coronary artery dissection in more proximal lesions rather than distal because of its less spatial resolution. It can rule out significant atherosclerotic disease, but sometimes if the patient has a non-calcified plaque, it can be mistaken as intramural hematoma, and CCTA approach can result in overdiagnosis [16].
MRI can also be useful to establish baseline because most patients with spontaneous coronary artery dissection have recurrent admissions with chest pain, in that case, a repeat MRI will be helpful to see if the patient actually had a new dissection event that will show as the new scar on MRI as compared to baseline.
Discussion
Management of acute SCAD
Spontaneous coronary artery dissection management depends upon several factors, including presenting clinical scenario (STEMI vs. NSTEMI), hemodynamically instability, and coronary anatomy features. Invasive coronary angiography is almost needed to establish the diagnosis, especially in patients who are presenting first time to the ER and do not have an established diagnosis of SCAD because of the lack of sensitivity of CCTA in detecting distal dissection as described earlier [16]. Intervention in spontaneous coronary dissection is challenging because of the presence of intramural hematoma and the risk of its expansion as compared to usual atherosclerotic disease presenting as an ACS.
Mostly expert consensus is the treatment in the presence of ACS should depend upon TIMI (Thrombolysis in Myocardial Infarction) flow. If the patient has a TIMI II or greater flow, conservative treatment should be considered since intervention in SCAD is challenging with a high rate of stent-related complications. Conservative therapy, especially in hemodynamically stable patients, has good outcomes, with studies showing spontaneous healing in most cases [17].
In hemodynamically unstable patients (i.e., cardiogenic shock, recurrent arrhythmias) or with TIMI flow of 0 or 1, immediate revascularization is required mainly by PCI (Percutaneous Coronary Intervention). Coronary Artery Bypass Graft (CABG) is usually needed if high-risk coronary artery anatomy features are present. High-risk features include dissection in left main, proximal LAD, and multivessel involvement; besides that, in other cases, PCI is reasonable if needed [2,9,18].
Regarding the specific percutaneous intervention technique, it is advisable to use a long stent to cover proximal and distal edges. Direct stenting without angioplasty also has been proposed to minimize propagation of dissection. On the contrary, some experts also suggested using balloon angioplasty first and if sufficient flow has been restored to avoid stenting because of long-term complications of revascularization associated with SCAD patients [19,20].
The major complication of PCI includes stent thrombosis, restenosis, and stent malposition. Due to these complications, the PCI success rate has been reported to be between 47% to 72% [9].
Nonetheless, SCAD was found to have early progression, so the first 5 to 7 days after index hospitalization is crucial. Risk factors associated with disease progression include intramural hematoma because that can block the vessels with pressure effect, involvement of LAD, stenosis >80%, and long lesion length [9].
Role of surgery
As discussed earlier, if the patient is found to have left main and proximal LAD involvement, then CABG should be considered. Because of advancements in percutaneous intervention, now very few patients have been referred for urgent CABG as bailout procedure. CABG has a high rate of initial revascularization, but like PCI does not prevent or protect against recurrent dissection events, plus long-term there is a high graft failure rate [21,22].
Role of medical therapy
Medical therapy, especially in stable patients, is the cornerstone of the treatment of SCAD. Medical therapy has been proven to show resolution of coronary dissection with complete healing of the vessel [17].
Medical therapy is discussed in detail as below.
Role of antiplatelet
Patients with SCAD should at least be on one antiplatelet, but if percutaneous intervention is performed; dual antiplatelet should be used according to the international society guidelines. In the absence of PCI, experts advocate using dual antiplatelet aspirin plus clopidogrel, especially in the acute phase of the event. Continuation of dual antiplatelet therapy for the long-term can be challenging because this disease much more predominantly affects women of younger age, and use of dual antiplatelet can cause menorrhagia, but single antiplatelet should be continued lifelong if tolerated [9,27].
Role of anticoagulation
Anticoagulation like heparin or GP IIb/IIIA should be avoided because of potential propagation of dissection due to expansion of intramural hematoma [24].
Role of Beta-blocker, ACE, and statin therapy
Among all medical therapy, beta-blocker is more efficacious. Lifelong beta-blocker treatment has been recommended. Studies also demonstrated that beta-blocker is also helpful in preventing recurrence of SCAD [24,25]. ACE inhibitors are also useful, especially if LVH or concomitant heart failure with reduced ejection fraction/LV failure is present. Antianginal like nitrates or amlodipine is also helpful to prevent chest pain.
Since SCAD is a non-atherosclerotic disease, use of statin is not beneficial, and data regarding it is conflicting; one smaller study shows higher recurrence rate in patients in which statin was used [14], but later large studies did not show any difference in recurrence rate between the patients who were on a statin [25]. The current recommendation is that statin use solely for SCAD is not indicated, but if patients have any primary prevention indication, i.e., diabetes, then statin should be used according to the guideline.
Long-term effect
Although SCAD is associated with a considerably higher rate of recurrent myocardial infarction, about 17 to 18%, overall mortality is <1% [26,27]. Factors that can precipitate recurrence include uncontrolled hypertension, migraine attack, and tortuous coronary artery vessels [25]. Therefore, to minimize recurrence, patients should take beta-blockers. ACE inhibitor/calcium channel blocker should be added if blood pressure is uncontrolled on the beta-blocker. In patients with migraines, triptans or other vasoconstrictive therapies should be avoided.
Role of cardiac rehabilitation
Patients post SCAD should have cardiac rehabilitation. SCAD specific rehab has an extremely specific protocol that includes a lower heart rate limit and blood pressure target during exercise. Patients should be discouraged and advised not to do high-intensity exercise, prolonged Valsalva maneuver, and hot yoga [26].
Pregnancy and SCAD
Although in pregnancy, the incidence of SCAD is rare, 1.8 cases per 100,000 pregnancies but it is one of the most important causes of acute myocardial infarction in the postpartum period. Peak incidence is usually around the third trimester and early postpartum period. Management is usually the same as in non-pregnant women. Medications like ACE inhibitors or statin should be avoided if SCAD happened in pregnancy or woman is breastfeeding. Beta-blocker carry some risk of intrauterine growth retardation during pregnancy, but specifically, atenolol should be avoided. Some experts' recommendation is against subsequent pregnancy. But nonetheless, subsequent pregnancy in diagnosed patients with SCAD should be considered high risk; therefore, before planning the next pregnancy, couples should have proper preconception counseling. Regarding contraception, non-hormonal contraception like IUD is preferred [27,28].
Conclusion
Spontaneous Coronary Artery Dissection disease (SCAD) was previously considered to be a rare disease now recognized as an important cause of acute myocardial infarctions, particularly in women that are young without risk factors for atherosclerotic disease, thanks to innovations in multimodality imaging. Medical management is the preferred mode of treatment, especially in hemodynamically stable patients with good outcomes. Long-term management is distinct from usual atherosclerotic disease and should be directed to prevent disease recurrence and improve quality of life.
Ethical Statement
The study was conducted in accordance with Helsinki Declaration as revised in 2013.
Conflict of Interest
None.
Funding
None.
Acknowledgments
None.
References
01. Pretty HC. Dissecting aneurysm of coronary artery in a woman aged 42. BMJ. 1: 667 (1931).
02. Adlam D, Alfonso F, Maas A, Vrints C, Al-Hussaini A, et al. European Society of Cardiology, acute cardiovascular care association, SCAD study group: A position paper on spontaneous coronary artery dissection. European Heart Journal. 39(36): 3353-3368 (2018).
03. Vanzetto G, Berger-Coz E, Barone-Rochette G, Chavanon O, Bouvaist H, et al. Prevalence, therapeutic management, and medium-term prognosis of spontaneous coronary artery dissection: Results from a database of 11,605 patients. Eur J Cardiothorac Surg. 35(2): 250-254 (2009).
04. Bush N, Nelson-Piercy C, Spark P, Kurinczuk JJ, Brocklehurst P, et al. Myocardial infarction in pregnancy and postpartum in the UK. Eur J Prev Cardiol. 20(1): 12-20 (2011).
05. Kearney P, Erbel R, Ge J, Zamorano J, Koch L, et al. Assessment of spontaneous coronary artery dissection by intravascular ultrasound in a patient with unstable angina. Cathet Cardiovasc Diagn. 32(1): 58-61 (1994).
06. Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, et al. Spontaneous coronary artery dissection. Circ Cardiovasc Interv. 7(5): 645-655 (2014).
07. Luong C, Starovoytov A, Heydari M, Sedlak T, Aymong E, et al. Clinical presentation of patients with spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 89(7): 1149-1154 (2017).
08. Lindor RA, Tweet MS, Goyal KA, Lohse CM, Gulati R, et al. Emergency department presentation of patients with spontaneous coronary artery dissection. J Emerg Med. 52(3): 286-291 (2017).
09. Lettieri C, Zavalloni D, Rossini R, Morici N, Ettori F, et al. Management and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol. 116(1): 66-73 (2015).
10. Nakashima T, Noguchi T, Haruta S, Yamamoto Y, Oshima S, et al. Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: A report from the angina pectoris-myocardial infarction multicenter investigators in Japan. Int J Cardiol. 207: 341-348 (2016).
11. Cheung CC, Starovoytov A, Parsa A, et al. In-hospital and long-term outcomes among patients with spontaneous coronary artery dissection presenting with ventricular tachycardia/fibrillation. Heart Rhythm 17(11): 1864-1869 (2020).
12. Fahmy P, Prakash R, Starovoytov A, Boone R, Saw J. Pre-disposing and precipitating factors in men with spontaneous coronary artery dissection. JACC Cardiovasc Interv. 9(8): 866-868 (2016).
13. Rogowski S, Maeder MT, Weilenmann D, Haager PK, Ammann P, et al. Spontaneous Coronary artery dissection: Angiographic follow-up and long-term clinical outcome in a predominantly medically treated population. Catheter Cardiovasc Interv. 89(1): 59-68 (2017).
14. Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 126(5): 579-588 (2012).
15. Jackson R, Al-Hussaini A, Joseph S, van Soest G, Wood A, et al. Spontaneous coronary artery dissection. JACC: Cardiovasc Imaging. 12(12): 2475-2488 (2019).
16. Eleid MF, Tweet MS, Young PM, Williamson E, Hayes SN, et al. Spontaneous coronary artery dissection: challenges of coronary computed tomography angiography. Eur Heart J Acute Cardiovasc Care 7(7): 609-613 (2017).
17. Hassan S, Prakash R, Starovoytov A, Saw J. Natural history of spontaneous coronary artery dissection with spontaneous angiographic healing. JACC: Cardiovasc Interv. 12(6): 518-527 (2019).
18. Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol. 68(3): 297-312 (2016).
19. Zghouzi M, Moussa Pacha H, Sattar Y, Alraies MC. Successful treatment of spontaneous coronary artery dissection with cutting balloon angioplasty. Cureus. 13(3): e13706 (2021).
20. Main A, Lombardi WL, Saw J. Cutting balloon angioplasty for treatment of spontaneous coronary artery dissection: Case report, literature review, and recommended technical approaches. Cardiovasc Diagn Ther. 9(1): 50-54 (2019).
21. Almeda FQ, Barkatullah S, Kavinsky CJ. Spontaneous coronary artery dissection. Clin Cardiol. 27(7): 377-380 (2004).
22. Tweet MS, Eleid MF, Best PJM, Lennon RJ, Lerman A, et al. Spontaneous coronary artery dissection. Circulation: Cardiovascular Interventions. 7(6): 777-786 (2014).
23. Alfadhli J, Alsharhan L, Martine C. Fibromuscular dysplasia presenting as spontaneous coronary artery dissection. J Cardiol Curr Res. 9(2): 00320 (2017).
24. Al-Hussaini A, Adlam D. Spontaneous coronary artery dissection. Heart. 103(13): 1043-1051 (2017).
25. Saw J, Humphries K, Aymong E, Sedlak T, Prakash R, et al. Spontaneous coronary artery dissection: Clinical outcomes and risk of recurrence. J Am Coll Cardiol. 70: 1148-58 (2017).
26. Chou AY, Prakash R, Rajala J, Birnie T, Isserow S, et al. The first dedicated cardiac rehabilitation program for patients with spontaneous coronary artery dissection: Description and initial results. Can J Cardiol. 32(4): 554-560 (2016).
27. Havakuk O, Goland S, Mehra A, Elkayam U. Pregnancy and the risk of spontaneous coronary artery dissection: An analysis of 120 contemporary cases. Circ Cardiovasc Interv. 10(3): e004941 (2017).
28. Azam MN, Roberts DH, Logan WF. Spontaneous coronary artery dissection associated with oral contraceptive use. Int J Cardiol. 48(2): 195-198 (1995).