- Open-Access Publishing
- Quality and Potential Expertise
- Flexible Online Submission
- Affordable Publication Charges
- Expertise Editorial Board Members
- 3 Week Fast-track Peer Review
- Global Visibility of Published Articles
The Role of Endoplasmic Reticulum Stress-Mediated Apoptosis in Vascular Calcification of Chronic Kidney Disease
Lin Jiang1, Lingling Wang2, Lingyun Wang3*, Chen Jiang1*
1Department of Nephrology, First Teaching Hospital of Tianjin University of Chinese Medicine, National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin 300384, China
2Department of Biostatistics, The University of Alabama at Birmingham, Birmingham, Alabama 35294, United States
3Division of Nephrology, Department of Medicine, The University of Alabama at Birmingham, Birmingham, Alabama 35294, United States
*Corresponding Author:
Chen Jiang, Department of Nephrology, First Teaching Hospital of Tianjin University of Chinese Medicine, National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin 300384, China, E-mail: jcdoctor_tcm@163.com
Lingyun Wang, Division of Nephrology, Department of Medicine, The University of Alabama at Birmingham, Birmingham, Alabama 35294, United States, E-mail: lingyunwang@uabmc.edu
Received date: July 19, 2021; Accepted date: August 16, 2021; Published date: August 23, 2021
Citation: Jiang L, Wang L, Wang L, Jiang C. (2021) The Role of Endoplasmic Reticulum Stress-Mediated Apoptosis in Vascular Calcification of Chronic Kidney Disease. Arch Neph Urol Stud. 1:02.
Copyright: © 2021 Jiang L. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Endoplasmic reticulum stress; Chronic kidney disease; Vascular calcification; Apoptosis
Abstract
Vascular calcification refers to the biological regulation process in which hydroxyapatite crystals are actively deposited between the extracellular matrix and arterial intima and media. It increases the risk of cardiovascular complications and death in chronic kidney disease. Chronic kidney disease is affected by the interaction of various physiological or pathological factors, which may lead to endoplasmic reticulum stress and the dysfunction of the endoplasmic reticulum. Endoplasmic reticulum stress plays an important role in apoptosis and is involved in the occurrence and development of vascular calcification. This article reviews the role of endoplasmic reticulum stress-mediated apoptosis in vascular calcification of chronic kidney disease.
Abbreviations :
4-PBA: 4-phenylbutyric acid; AKI: Acute Kidney Injury; ALP: alkaline phosphatase; ASK1: Apoptotic Signal-regulated Kinase 1; ATF4/6: Activated Transcription Factor 4/6; Bad: B-cell leukemia/lymphoma-2-associated death promoter homolog; Bcl-2: B-cell lymphoma 2; Bcl-xl: B-cell lymphoma xl; Bim: Bcl-2 interaction mediator of cell death; cAMP/PKA: cyclic adenylate monophosphate/protein kinase A; CHOP: CCAAT Enhancer Binding Protein (C/EBP) homologous protein; CIHH: Chronic Intermittent Hypobaric Hypoxia; CKD: Chronic Kidney Disease; DN: Diabetic Nephropathy; DR5: Death Receptor 5; EPO: erythropoietin; ERS: Endoplasmic Reticulum Stress; FGF21: Fibroblast Growth Factor 21; GRP78: 78-kDa Glucose-Regulated Protein; Hcy: homocysteine; IMD: intermedin; IRE1: Inositol-Requiring Enzyme 1; JNK: C-Jun N-terminal kinase; MAPK: Mitogen-Actived Protein Kinase; mTORC1: mammalian Target of Rapamycin Complex 1; PERK: Protein ER Kinase (PKR)-like ER kinase; PPARγ: Peroxisome Proliefators-Activated Receptor γ; PUMA: p53 up-regulated modulator of apoptosis; Runx2: runt-related transcription factor 2; TAU: taurine; TNFα: Tumor Necrosis Factor-α; TRAF2: tumor necrosis factor receptor-associated factor 2; UPR: Unfolded Protein Response; VC: Vascular Calcification; VSMC: Vascular Smooth Muscle Cell.
Introduction
Vascular Calcification (VC), as a common feature of Chronic Kidney Disease (CKD), increases the risk of cardiovascular disease and death in CKD. Vascular calcification refers to the biological regulation process in which hydroxyapatite crystals are actively deposited between the extracellular matrix and arterial intima and media. Vascular calcification includes intimal calcification, medial calcification, valve calcification, and other pathological types. The molecular pathogenesis of vascular calcification in CKD is extremely complicated and is affected by the interaction of many factors such as calcium and phosphorus metabolism disorders, inflammation, oxidative stress, apoptosis, endoplasmic reticulum stress, etc. [1].
Endoplasmic Reticulum Stress (ERS) can regulate the occurrence and development of VC by promoting the Unfolded Protein Response (UPR) to induce the osteogenic phenotype transformation, apoptosis, and autophagy of Vascular Smooth Muscle Cells (VSMCs) [2,3]. This article reviews the role of ERS-mediated apoptosis in vascular calcification of CKD, which may help to provide new ideas to prevent and treat vascular calcification.
Literature Review
Endoplasmic reticulum stress
Endoplasmic reticulum is an important organelle to maintain the protein homeostasis of cells. It is widely distributed in cells, and the membrane area of it accounts for 50% of all membrane structures. It is the main site for protein synthesis, protein processing and transport, lipid synthesis, and intracellular Ca2+ storage [4].
Endoplasmic reticulum homeostasis is maintained through a series of coordinated adaptive responses. Many physiological and pathological factors [4], such as hyperglycemia, hyperlipidemia, hypoxia, acidosis, and calcium balance disorder, can cause the dysfunction and dyshomeostasis of endoplasmic reticulum, which finally leads to ERS. ERS is a protective mechanism of cells, which is manifested by the accumulation of a large number of unfolded or misfolded proteins in the endoplasmic reticulum cavity and by the disorder of Ca2+ balance. Under normal circumstances, cells protect the function of ERS and prevent its further damage by reducing the translation of target proteins, inducing the synthesis of molecular chaperone, and activating protein degradation related to ER. This process is called Unfolded Protein Reaction (UPR) [5], which is a signal transduction cascade to limit the accumulation of unfolded proteins and to restore ER homeostasis. However, when ERS is severe, the UPR can cause apoptosis.
UPR is mainly mediated by three ER stress receptors: Protein ER kinase (PKR)-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1), and Activated Transcription Factor 6 (ATF6) [6]. Under physiological conditions, ER stress receptors are in an inactivated state through the association with ER chaperone 78-kDa Glucose-Regulated Protein (GRP78). When ERS occurs, GRP78 will be separated from the receptors and combined with the unfolded protein to activate the three classic signaling pathways of PERK, IRE1, and ATF6 (The details of the three signaling pathways are described in Discussion section). This would promote the correct folding of the protein, inhibit the production of the protein, accelerate the degradation of error protein, and enhance the self-repair ability of endoplasmic reticulum [7,8]. This means that a certain degree of ERS is conducive to activating the protective adaptation mechanism of cells.
However, once ERS is too strong or lasts for too long, it exceeds the compensation capacity of ER. This makes a serious imbalance of the ER steady state in which the function of ER is unable to be repaired and ultimately leads to the activation of apoptotic signaling pathway [9,10].
ERS in kidney disease
It is reported that ERS is involved in the occurrence and development of a variety of diseases. Recent studies have shown that ERS-mediated apoptosis is associated with kidney diseases and it is an important pathogenesis for kidney diseases including Acute Kidney Injury (AKI) and CKD.
Proteinuria caused by hyperglycemia-induced podocyte injury is one of the main factors leading to the progressive development of Diabetic Nephropathy (DN). There are evidences that ERS causes apoptosis of mouse podocytes through different mechanisms and participates in the development process of DN [11]. The injury and death of renal tubular epithelial cells are the pathological characteristics of AKI. Studies have indicated that ERS is continuously activated in a mouse model of AKI-CKD transition after unilateral renal ischemia-reperfusion injury, and the inhibition of ERS significantly reduces tubular atrophy and renal fibrosis [12,13]. The induction of ERS may be cytoprotective, but it can also be cytotoxic by activating apoptosis, causing cell damage and leading to the apoptosis of a series of renal cells, such as podocytes, renal tubular cells, glomerular endothelial cells, and mesangial cells, which could finally result in the occurrence of glomerular sclerosis and interstitial fibrosis [14]. ERS promotes apoptosis of VSMCs through URP in the progression of CKD and triggers vascular calcification [2]. Therefore, ERS plays a vital role in the initiation and continuation of CKD through different pathways.
Discussion
ERS induces apoptosis and promotes vascular calcification
ERS can promote VC by inducing apoptosis of VSMCs [15,16]. The pathways involved in apoptosis include PERK/eIF2α/CHOP pathway, IRE1-mediated C-Jun N-terminal kinase (JNK) pathway, and caspase-12 pathway (Figure 1).
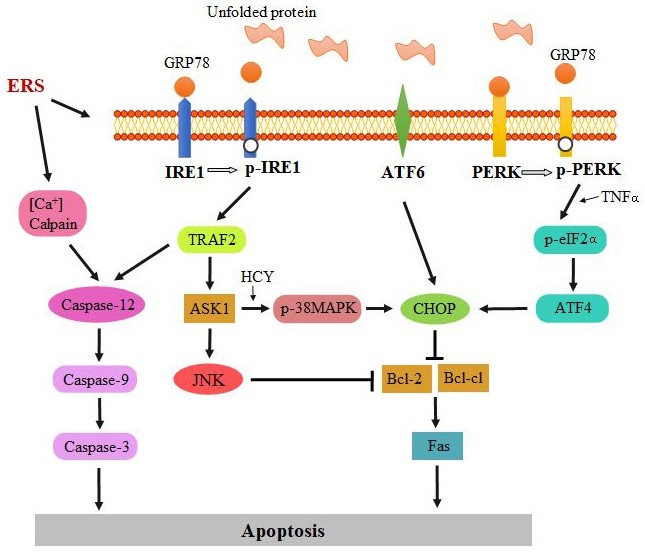
Figure 1: The pathways that are involved in ERS-mediated apoptosis: PERK/eIF2α/CHOP pathway, IRE1-mediated JNK pathway, and caspase-12 pathway.
PERK/eIF2α/CHOP pathway: The CCAAT Enhancer Binding Protein (C/EBP) homologous protein (CHOP, also known as growth arrest and DNA damage-inducible gene 153, is an ERS-specific transcription factor [17]. It plays a crucial role in the pathogenesis of CKD-dependent vascular calcification [18]. Though all the three classic pathways can induce the expression of CHOP, the PERK-eIF2α-ATF4 pathway is the main pathway for CHOP expression [19].
PERK is a protein kinase that belongs to eIF2α kinase family. It contains two domains: A cytoplasmic domain and a kinase domain. The cytoplasmic domain is responsible for the accumulation of unfolded/misfolded proteins in the ER lumen. In the early stage of UPR, PERK is activated by the autophosphorylation of its kinase domain (p-PERK), which could phosphorylate eIF2α (Figure 1). By phosphorylating B-cell leukemia/lymphoma-2-associated death promoter homolog (Bad), the levels of B-cell lymphoma xl (Bcl-xl) and B-cell lymphoma 2 (Bcl-2) proteins with anti-apoptotic activity are increased. In addition, the expression of Fas with a pro-apoptotic effect is decreased, and the sensitivity of apoptosis regulatory factor Fas to induce apoptosis is reduced, which promotes the generation of anti-apoptotic effect. However, when severe or long-term ERS occurs, phosphorylated eIF2α enhances the translation of ATF4, thereby up-regulating the expression of CHOP gene and increasing the expression of pro-apoptotic related genes [such as Bcl-2 interaction mediator of cell death (Bim), Death Receptor 5 (DR5), and p53 up-regulated modulator of apoptosis (PUMA)], as well as reducing the expression of the anti-apoptotic gene Bcl-2, which eventually leads to apoptosis [20].
ATF4 is a key transcription factor, which not only regulates UPR/ERS during bone formation but also regulates osteoblast differentiation. Activation and induction of ATF4 are the key events in the pathogenesis of stearate-induced vascular calcification. Stearate and its metabolites can activate the PERK-eIF2 pathway of UPR, increase the level of phosphorylated eIF2α, induce the translation of ATF4 in VSMCs, and participate in the osteoblast differentiation and mineralization and ERS-mediated VSMCs apoptosis [21].
In addition, the PERK-eIF2α-ATF4-CHOP axis activated by Tumor Necrosis Factor-α (TNFα) may also participate in the occurrence of vascular calcification. TNFα is a major inflammatory cytokine. The serum TNFα levels have been significantly increased in patients with CKD. Previous studies have shown that TNFα is a key mediator of vascular calcification [22,23]. TNFα can also activate two protein kinases, PKA and RSK2, to induce ATF4 phosphorylation through PKA and MEK-ERK-RSK 2 pathways. ATF4 activates CHOP expression and induces the type III sodium-dependent phosphate transporter (Pit-1), which causes an increase of inorganic phosphate uptake and induces osteoblastic differentiation and the mineralization of VSMCs [24].
The research data from Panda, et al. show that components of the uremic state [activation of the receptor for advanced glycation end products (RAGE) and hyperphosphatemia] induce increased or prolonged activity of mammalian Target of Rapamycin Complex 1 (mTORC1), which leads to ERS disorder. ATF4-CHOP mediates cell apoptosis, inhibits the production of effective mineralization inhibitor pyrophosphate, and enhances the transdifferentiation of VSMCs into osteochondral phenotype, thereby increasing vascular calcification [25].
IRE1-mediated JNK pathway: The c-Jun N-terminal kinase (JNK) and p38MAPK are important members of the mitogen-activated protein kinase (MAPK) family, which regulate cell apoptosis through various channels [26]. The JNK pathway is activated by the IRE1-TRAF2-ASK1 branch of UPR.
IRE1 is a key UPR signal activator that responds to ERS by propagating the UPR signal from the ER to the cytosol. Like PERK, IRE1 can also be activated by its autophosphorylation (p-IRE1) from the disassociation of GRP78. IRE1 can directly act on tumor necrosis factor receptor-associated factor 2 (TRAF2) and activate apoptotic signal-regulated kinase 1 (ASK1), which promotes the phosphorylation of JNK and p38 MAPK [27]. The phosphorylated JNK promotes the expression of pro-apoptotic genes BID and BIM in the Bcl-2 family, and inhibits the expression of anti-apoptotic genes Bcl-2, Bcl-xl, and MCL-1. Furthermore, the phosphorylation of p38 MAPK activates CHOP and promotes the expression of BIM and DR5, which can induce apoptosis. Research study has also confirmed that p38 MAPK pathway is involved in high glucose-induced vascular calcification by activating ERS and apoptosis [28].
Homocysteine (Hcy) is a sulfur-containing amino acid formed after the demethylation of methionine. Hyperhomocysteinemia, in which Hcy level is greater than 15 μmol/L, can cause a variety of cardiovascular diseases. Zou, et al. demonstrated that Hcy increases the activity of NADPH oxidase in VSMCs by activating p38 MAPK and enhancing the phosphorylation of p47phox [29]. Hou, et al. found that Hcy promotes vascular calcification by activating ERS on a rat VSMCs calcification model cultured in vitro. This suggests that Hcy may induce ERS to promote calcification through the p38 MAPK pathway [30].
Caspase-12 pathway: Caspase-12 pathway is the unique apoptosis pathway of ERS. Caspase-12 belongs to the caspase family, which is located on the endoplasmic reticulum membrane in the form of inactive zymogen under physiological conditions. When ERS occurs, caspase-12 specific cleavage is activated, but it is not activated by other apoptotic signals. It directly activates caspase-9 and then activates caspase-3, which leads to cell apoptosis [31]. Caspase-12 can be activated by TRAF2 through IRE1 pathway [32], and it can be produced by calpain through the cleavage of procaspase-12 (precursor of caspase-12) [33]. It was found that caspase-12-deficient mice could resist apoptosis induced by ERS, while other death stimuli still promoted apoptosis. This indicated that caspase-12 is related to the mechanism of ERS-mediated apoptosis, but not to non-ERS-mediated apoptosis [34,35]. Therefore, caspase-12 is often used to detect the presence of ERS-induced apoptosis in the study of vascular calcification.
Pioglitazone, as a thiazolidinedione hypoglycemic agent, has been widely used in clinical diabetes treatment. Activation of Peroxisome Proliefators-Activated Receptor γ (PPARγ) regulates the transcription of insulin-related genes involved in glucose and lipid metabolism. Results have shown that after treatment with pioglitazone, the mRNA levels of ERS indicators GRP78 and caspase-12 are significantly increased, and vascular smooth muscle calcification is aggravated. These results indicate that pioglitazone could promote the expression of PPARγ and act on endoplasmic reticulum. The up-regulation of GRP78 and caspase-12 causes excessive ERS and aggravates cell calcification [36].
Methods to Treat Vascular Calcification by Regulating ERS
ERS inhibitors
4-phenylbutyric acid (4-PBA) is a chemical partner that can stabilize the protein conformation, improve the folding ability, and inhibit ERS. It can reduce the accumulation and aggregation of misfolded proteins in the endoplasmic reticulum lumen and restore the homeostasis of the endoplasmic reticulum [37]. After Zhu, et al. treated rats with VC using 4-PBA, the protein levels of ERS markers GRP78, CHOP, and caspase-12 were significantly decreased, and the expression of osteogenic marker runt-related transcription factor 2 (Runx2) and the protein level of alkaline phosphatase (ALP) activity were lowered, which indicated that 4-PBA can reduce ERS-mediated apoptosis and inhibit the phenotypic transformation of VSMCs into osteoblast-like cells and delay the progress of vascular calcification [16,38].
Taurine (TAU), as a chemical chaperone or inhibitor of ERS, can regulate calcium homeostasis, stabilize the mutated protein, and promote protein folding to reduce ERS. Treatment of calcified rats with TAU can decrease the number of apoptotic VSMCs and the cleaved caspase-12 protein level. It also reduces the calcium content, ALP activity, and calcium deposition in calcified aortas, thus preventing the phenotypic transformation of VSMCs. This indicates that TAU inhibited apoptosis and VC development by reducing ERS [38,39].
Cardiovascular protective factor
Intermedin (IMD) is a member of Calcitonin Gene-Related Peptide (CGRP) family, which has a protective effect on the heart [40]. Its main pharmacological action is to activate cyclic adenylate monophosphate/protein kinase A (cAMP/PKA) signal. In a study of rat VSMCs, IMD1-53 reversed the up-regulation of protein levels of ERS markers in calcified VSMCs, reduced the apoptosis of calcified VSMCs, delayed the osteoblastic-like transformation of contracted VSMCs, and reduced the calcification of VSMCs [41].
Fibroblast growth factor 21 (FGF21), a new member of FGF family, has biological effects such as anti-inflammation, anti-oxidation, lipid regulation, and reducing insulin resistance. It is a protective factor for the cardiovascular system. It can inhibit vitamin D3 and nicotine-induced vascular calcification, apoptosis, and gene expression of osteogenic marker and ERS marker [42]. The FGF21 treatment is involved in CHOP and caspase-12 pathway but not JNK pathway [43].
Erythropoietin (EPO) is a major factor regulating erythropoiesis in mammals and is mainly secreted in adult kidney. As the kidney parenchyma continues to decrease in CKD patients, the synthesis of EPO also decreases, and symptoms of anemia appear. In the study of Chang, et al., EPO treatment of rats with CKD or calcified VSMCs reduced the protein levels of ATF4 and GRP94 and reversed CHOP and caspase-12-mediated ERS-induced apoptosis. Therefore, EPO exerts a variety of tissue-protective effects by inhibiting ERS and apoptosis, thereby reducing VC. EPO can be used to treat the anemia in patients with CKD and used as a target of VC treatment. However, attention should be paid to the selection of EPO types and doses during the use of EPO to prevent complications such as thrombosis [44].
Non-drug therapy
Chronic Intermittent Hypobaric Hypoxia (CIHH) is an intermittent simulation of high altitude hypobaric hypoxia, with important cardiovascular protection and vasodilation functions. CIHH can significantly reduce the calcium content and ALP activity in the aortic tissue of vitamin D3 plus nicotine (VDN) rats, and increase the down-regulation of the expression of contraction phenotypic marker proteins in aortic smooth muscle cells, inhibit the up-regulation of phenotypic marker proteins in osteoblasts, and inhibit the increase of ERS marker proteins GRP78, CHOP, and caspase-12, thereby improving vascular calcification [45].
Conclusion
Damage and apoptosis of vascular endothelial cells are often the starting point of vascular disease. ERS can protect the endoplasmic reticulum homeostasis and make the cells survive, however, excessive ERS can lead to apoptosis. ERS is affected by a variety of pathophysiological factors. Regulating ERS to fight against vascular endothelial cell apoptosis, using drugs to maintain endoplasmic reticulum homeostasis, and treating or delaying vascular calcification in CKD have become new ideas and targets for the current treatment. It is believed that with the deepening of research, there will be new breakthroughs and progress in the effective treatment of chronic kidney disease in the future.
References
01. Shroff R, Long DA, Shanahan C. Mechanistic insights into vascular calcification in CKD. J Am Soc Nephrol. 24(2): 179-189 (2013).
02. Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, et al. Apoptosis regulates human vascular calcification in vitro: Evidence for initiation of vascular calcification by apoptotic bodies. Circ Res. 87(11): 1055-1062 (2000).
03. Duan X, Zhou Y, Teng X, Tang C, Qi Y. Endoplasmic reticulum stress-mediated apoptosis is activated in vascular calcification. Biochem Biophys Res Commun. 387(4): 694-699 (2009).
04. Inagi R, Ishimoto Y, Nangaku M. Proteostasis in endoplasmic reticulum-new mechanisms in kidney disease. Nat Rev Nephrol. 10(7): 369-378 (2014).
05. Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 529(7586): 326-335 (2016).
06. Walter P, Ron D. The unfolded protein response: From stress pathway to homeostatic regulation. Science. 334(6059): 1081-1086 (2011).
07. Karagöz GE, Acosta-Alvear D, Nguyen HT, Lee CP, Chu F, et al. An unfolded protein-induced conformational switch activates mammalian IRE1. Elife. 6: e30700 (2017).
08. Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 5(5): 897-904 (2000).
09. Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 13(3): 184-190 (2011).
10. Woehlbier U, Hetz C. Modulating stress responses by the UPRosome: A matter of life and death. Trends Biochem Sci. 36(6): 329-337 (2011).
11. Cao Y, Hao Y, Li H, Liu Q, Gao F, et al. Role of endoplasmic reticulum stress in apoptosis of differentiated mouse podocytes induced by high glucose. Int J Mol Med. 33(4): 809-816 (2014).
12. Shu S, Zhu J, Liu Z, Tang C, Cai J, et al. Endoplasmic reticulum stress is activated in post-ischemic kidneys to promote chronic kidney disease. EBioMedicine. 37: 269-280 (2018).
13. Ricciardi CA, Gnudi L. The endoplasmic reticulum stress and the unfolded protein response in kidney disease: Implications for vascular growth factors. J Cell Mol Med. 24(22): 12910-12919 (2020).
14. Cybulsky AV. Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat Rev Nephrol. 13(11): 681-696 (2017).
15. Hetz C, Chevet E, Oakes SA. Proteostasis control by the unfolded protein response. Nat Cell Biol. 17(7): 829-838 (2015).
16. Zhu Q, Guo R, Liu C, Fu D, Liu F, et al. Endoplasmic reticulum stress-mediated apoptosis contributing to high glucose-induced vascular smooth muscle cell calcification. J Vasc Res. 52(5): 291-298 (2016).
17. Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18(24): 3066-3077 (2004).
18. Shinobu M, Masuda M, Demos-Davies KM, Keenan AL, Saunders SJ, et al. Endoplasmic reticulum stress effector CCAAT/enhancer-binding protein homologous protein (CHOP) regulates chronic kidney disease-induced vascular calcification. J Am Heart Assoc. 3(3): e000949 (2014).
19. Li Y, Guo Y, Tang J, Jiang J, Chen Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin (Shanghai). 46(8): 629-640 (2014).
20. Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 7(9): 880-885 (2006).
21. Masuda M, Ting TC, Levi M, Saunders SJ, Miyazaki-Anzai S, et al. Activating transcription factor 4 regulates stearate-induced vascular calcification. J Lipid Res. 53(8): 1543-1552 (2012).
22. Tintut Y, Patel J, Parhami F, Demer LL. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation. 102(21): 2636-2642 (2000).
23. Chasseraud M, Liabeuf S, Mozar A, Mentaverri R, Brazier M, et al. Tumor necrosis factor-related apoptosis-inducing ligand and vascular calcification. Ther Apher Dial. 15(2): 140-146 (2011).
24. Masuda M, Miyazaki-Anzai S, Levi M, Ting TC, Miyazaki M. PERK-eIF2α-ATF4-CHOP signaling contributes to TNFα-induced vascular calcification. J Am Heart Assoc. 2(5): e000238 (2013).
25. Panda DK, Bai X, Sabbagh Y, Zhang Y, Zaun H, et al. Defective interplay between mTORC1 activity and endoplasmic reticulum stress-unfolded protein response in uremic vascular calcification. Am J Physiol Renal Physiol. 314(6): F1046-F1061 (2018).
26. Liang X, Yang K, Caspase, JNK/SAPK, p38 MAPK and cell apoptosis. Foreign Medical Sciences-Section Hygiene. 35: 5-10 (2008).
27. Ron D, Hubbard S. How IRE1 reacts to ER stress. Cell. 132(1): 24-26 (2008).
28. Guo R, Liu C, Wu Z, Jiang J, Wu B, et al. Involvement of p38 MAPK-activated endoplasmic reticulum stress in high glucose-induced calcification of vascular smooth muscular cells. J Guangdong Medical College. 34: 225-229 (2016).
29. Zou T, Yang W, Hou Z, Yang J. Homocysteine enhances cell proliferation in vascular smooth muscle cells: role of p38 MAPK and p47phox. Acta Biochim Biophys Sin (Shanghai). 42(12): 908-915 (2010).
30. Hou Y, Lu W, Zhang J, Ni X, Yu Y, et al. Homocysteine exacerbates rat vascular smooth muscle cells calcification by activating endoplasmic reticulum stress. Chinese J Arteriosclerosis. 23: 437-442 (2015).
31. Wang X, Shao X, Xu H, Ye L, Xu X, et al. Effects Tangzhiqing on the expression of endoplasmic reticulum stress related factors GRP78, CHOP and Caspase-12 in the hippocampus of diabetic rats. J Nanjing University of Traditional Chinese Medicine. 35: 73-77 (2019).
32. Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, et al. Activation of caspase-12, an Endoplastic Reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem. 276(17): 13935-13940 (2001).
33. Nakagawa T, Yuan J. Cross-talk between two cysteine protease families: Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 150(4): 887-894 (2000).
34. Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 29(1): 42-61 (2008).
35. Nakagawa T, Zhu H, Morishima N, Li E, Xu J, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 403(6765): 98-103 (2000).
36. Ma Q, Bian Y, Bai R, Lu Y, Xiao C. Pioglitazone increases calcification of rat vascular smooth muscle cells by endoplasmic reticulum stress apoptotic pathway. Guide of China Medicine. 13: 13-16 (2015).
37. Kolb PS, Ayaub EA, Zhou W, Yum V, Dickhout JG, et al. The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int J Biochem Cell Biol. 61: 45-52 (2015).
38. Duan X, Chuang J, Zhang J, Zhang B, Li Y, et al. Activating transcription factor 4 is involved in endoplasmic reticulum stress-mediated apoptosis contributing to vascular calcification. Apoptosis. 18(9): 1132-1144 (2013).
39. Das J, Ghosh J, Manna P, Sil PC. Taurine protects rat testes against doxorubicin-induced oxidative stress as well as p53, Fas and caspase 12-mediated apoptosis. Amino Acids. 42(5): 1839-1855 (2012).
40. Yang J, Jia Y, Pan C, Zhao J, Ouyang M, et al. Effects of intermedin(1-53) on cardiac function and ischemia/reperfusion injury in isolated rat hearts. Biochem Biophys Res Commun. 327(3): 713-719 (2005).
41. Chang J, Duan X, Zhang B, Teng X, Zhou Y, et al. Intermedin1-53 attenuates vascular smooth muscle cell calcification by inhibiting endoplasmic reticulum stress via cyclic adenosine monophosphate/protein kinase A pathway. Exp Biol Med (Maywood). 238(10): 1136-1146 (2013).
42. Jin H, Liu L, Gao B. The role of endoplasmic reticulum stress in the inhibition of vascular calcification by fibroblast growth factor 21 in rats. Chinese J Arteriosclerosis. 27: 1032-1036 (2019).
43. Shi Y, Wang S, Peng H, Lv Y, Li W, et al. Fibroblast growth factor 21 attenuates vascular calcification by alleviating endoplasmic reticulum stress mediated apoptosis in rats. Int J Biol Sci. 15(1): 138-147 (2019).
44. Chang J, Sun N, Liu Y, Wei M, Zhao Y, et al. Erythropoietin attenuates vascular calcification by inhibiting endoplasmic reticulum stress in rats with chronic kidney disease. Peptides. 123: 170181 (2020).
45. Li Y, Yang R, Jin S, Yuan F, Wu Y, et al. Chronic intermittent hypobaric hypoia ameliorates vascular calcification via inhibiting endoplasmic reticulum stress in rats. Chinese J Arteriosclerosis. 23: 443-447 (2015).