- Open-Access Publishing
- Quality and Potential Expertise
- Flexible Online Submission
- Affordable Publication Charges
- Expertise Editorial Board Members
- 3 Week Fast-track Peer Review
- Global Visibility of Published Articles
Ocular manifestations of Zika virus: A systematic review
Carrie Yuen Ling Chan1, Sunny Chi Lik Au2*
1Department of Out-patient Clinic, Hong Kong East Cluster, Hospital Authority, Hong Kong, China
2Department of Ophthalmology, Tung Wah Eastern Hospital, Hong Kong, China
*Corresponding Author: Sunny Chi Lik Au, Department of Ophthalmology, Tung Wah Eastern Hospital, Hong Kong, China, E-mail: kilihcua@gmail.com
Received date: May 17, 2021; Accepted date: June 03, 2021; Published date: June 10, 2021
Citation: Chan CYL, Au SCL (2021) Ocular manifestations of Zika virus: A systematic review. J Ophthalmol Eye Dis Tre. 1: 01.
Copyright: © 2021 Chan CYL. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Zika virus, Ocular, Optic nerve, Uveitis, Cornea
Abstract
Introduction: A recent endemic outbreak of Zika virus infection in newborn poses serious public health concern because of its associated complications such as microcephaly. Reports also suggest ocular complications associated with these diseases, but its findings are still limited. We therefore conduct this systematic review to summarize the ocular manifestations of Zika Virus.
Method: PubMed, OvidSP, EMBASE were searched for reports on Zika virus related ocular findings. Case series, reports and cohorts were identified from reference list of included studies. Studies with overlapped subjects were excluded. Clinical findings data were extracted, categorized and summarized to study the spectrum of ocular findings associated with Zika virus infection. Incidence was calculated.
Results: Twelve studies including a total of 51 infant cases with 84 affected eyes and 3 adult cases with 5 affected eyes were included for analysis. Macular pigment mottling (55.9%), foveal reflex loss (41.2%), chorioretinal atrophy (27.4%), optic nerve hypoplasia (20.6%) and increased cup-disc ratio (18.6%) were common ocular findings related to Zika virus in infants. Uveitis and maculopathy were seen in adult cases.
Conclusion: Macular and optic nerve abnormalities are the major ocular findings in Zika virus infected infants. Eventual visual outcome is still unknown. Screening guidelines have been established. Further studies on Zika virus related ocular findings are of paramount importance for the global public health.
Introduction
Zika virus is a mosquito-borne virus [1], which can be transmitted through intrauterine route, sexual intercourse, bite by infected species of Aedes mosquitoes, blood transfusion or laboratory exposure [2-8]. The epidemic outbreak of Zika virus in Brazil during year 2015 to 2016 poses serious public health concern globally, as the infection caused a dramatic increase in incidence of microcephaly [1]. The possible ocular abnormalities related to Zika virus was then studied and was first reported in January of 2016. [9] The exact pathogenesis of how Zika virus causing damage to ocular structures remains unclear. It was proposed that Zika virus could spread along nerves or in blood to eyes and the resulted damage could be direct viral damage or through inflammatory response.
The aim of our study is to summarize Zika virus related ocular abnormalities that were reported in the currently available literature and discuss its prevalence, pathogenesis, risk factors, prognosis, treatment, prevention, and screening.
Methods
Eligibility criteria
Cohort studies, cases series and case reports that reported ocular features in patients with either presumed or confirmed Zika virus infection were included. Publications other than English-language would be excluded. Reviews, guidelines, pathology and animal studies, commentaries, news, and hypotheses papers were all excluded.
Search strategy
PubMed, OvidSP, EMBASE were searched from their inception to 27th November 2016. Keywords used were “Zika” and (“eye” or “ocular” or “macula” or” retina” or “optic nerve” or “anterior segment” or “uveitis “or ‘cornea” or “iris” or “lens” or “choroidal” or “vitreous”). Titles and abstracts were screened to exclude non-eligible studies. Full texts of remaining studies were reviewed and excluded accordingly. Reference lists of the all these articles were further screened for possible eligible studies. Studies published by the same research group, or same first author would be cross-referenced to eliminate double representation of the same subject. For studies recruiting same subjects, only the one with larger sample size would be included.
Data extraction
Demographic data and ocular related findings were reviewed and extracted. These data includes locality of studies, number of patients involved, number of eyes affected, demographics of subjects, and method of diagnosis. In the aspect of ocular findings of subjects, bilateral or unilateral involvement, laterality of the affected eye, anatomical site of the abnormalities, and nature of the abnormalities were all extracted.
Data analysis
Infant and adult subjects were analyzed separately. All the reported infant cases with clinical ocular findings were listed out in table format. The corresponding ocular clinical findings were then categorized into anatomical subgroups and prevalence of each ocular finding was calculated with the formula (number of eyes with that feature divided by total number of eyes included). Total number of eyes included is equal to total number of infants affected times 2, assuming each affected infant has 2 eyes. Further subgroup analysis based on the head circumference would be attempted. Ophthalmologic imaging findings were described separately.
Results
Study selection and characteristics
50 studies were identified from the 3 databases after excluding overlapping studies, and 14 were excluded by screening the titles and abstracts. 36 full text articles were reviewed and 24 were further excluded according to our inclusion and exclusion criteria. Finally 12 articles were included [9-20]. Figure 1 shows the flow chart of the selection process of the included articles, and with reasons for those excluded. There were multiple studies reported by the same research group and of the same first author by Ventura [9,10,14-17]. These studies showed no overlapping of subjects in terms of clinical ocular findings, thus were all included. Therefore, study numbers ranging from 1 to 12 were assisted to each included studies for better referencing of studies in paragraph of this review. Table 1 listed out their respective referencing number, and the characteristics of each included articles.
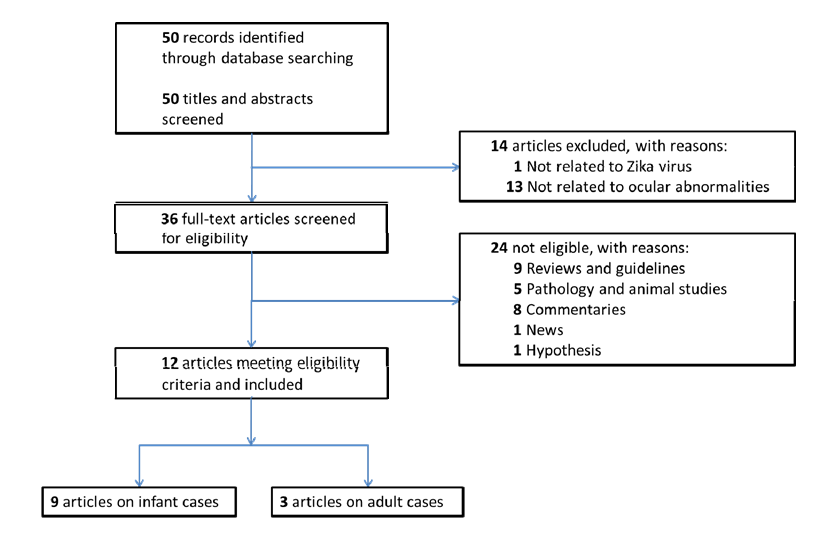
Figure 1: Flow Chart showing the selection process of the included articles.
| Study no. | Studies/articles | Location | No. of patients | No. of eyes affected | Mean age (Range) | Male (M): Female (F) | Inclusion criteria | Zika virus infection | Ocular findings |
| 1 | Ventura et al, 2016 [9] | Brazil | 3 | 3 | NA | NA | Microcephaly | Presumed | Clinical |
| 2 | Ventura et al, 2016 [10] | Brazil | 10 | 17 | 1.9 (0.7-2.9 months) | 4M 6F | Microcephaly | Presumed | Clinical |
| 3 | Freitas et al, 2016 [11] | Brazil | 10 out of 29 | 17 | NA (1-6 months) | NA | Microcephaly | Presumed | Clinical |
| 4 | Culjat et al, 2016 [12] | Brazil | 1 | 2 | Day 5 | M | NA | Confirmed by ZIKV IgM Ab | Clinical |
| 5 | de Miranda et al, 2016 [13] | Brazil | 3 | 6 | NA | 3M | Microcephaly | Presumed | Clinical |
| 6 | Ventura et al, 2016 [14] | Brazil | 22 out of 40 | 37 | 2.2 (0.1-7.3 months) | 9M 13F | Microcephaly | 24/40 confirmed by ZIKV | Clinical |
| 7 | Ventura et al, 2016 [15] | Brazil | 1 | 1 | Day 57 | NA | NA | Confirmed by ZIKV IgM Ab | Clinical |
| 8 | Ventura et al, 2016 [16] | United States | 1 | 1 | Day 6 | F | NA | Confirmed by ZIKV IgM Ab | Clinical |
| 9 | Ventura et al, 2016 [17] | Brazil | 8 | 11 | 4.1 (3-5.1 months) | 3M 5F | With previous diagnosis | 7/8 confirmed by ZIKV IgM Ab | OCT |
| 10 | Fontes, 2016 [18] | Brazil | 1 | 2 | 39 years old | M | NA | Presumed | Clinical |
| 11 | Furtado et al, 2016 [19] | Brazil | 1 | 2 | Early 40s | M | NA | Confirmed by ZIKV RNA in right | Clinical |
| 12 | Parke et al, 2016 [20] | United States | 1 | 1 | 64 years old | M | NA | Confirmed by PRNT assay | Clinical, OCT, FA |
| Abbreviations: OCT: Optical Coherence Tomography; FA: Fluorescein Angiogram; NA: Not Available | |||||||||
Table 1: Characteristics of included articles.
Study no. 9 by Ventura et al. [17] included some of the patients that were described in other included studies. [9,10,14,15] However, this article reported only the Optical Coherence Tomography (OCT) findings but not clinical findings. Therefore, the affected patients or eyes in this case series were described separately from the summary of the clinical findings (Table 2).
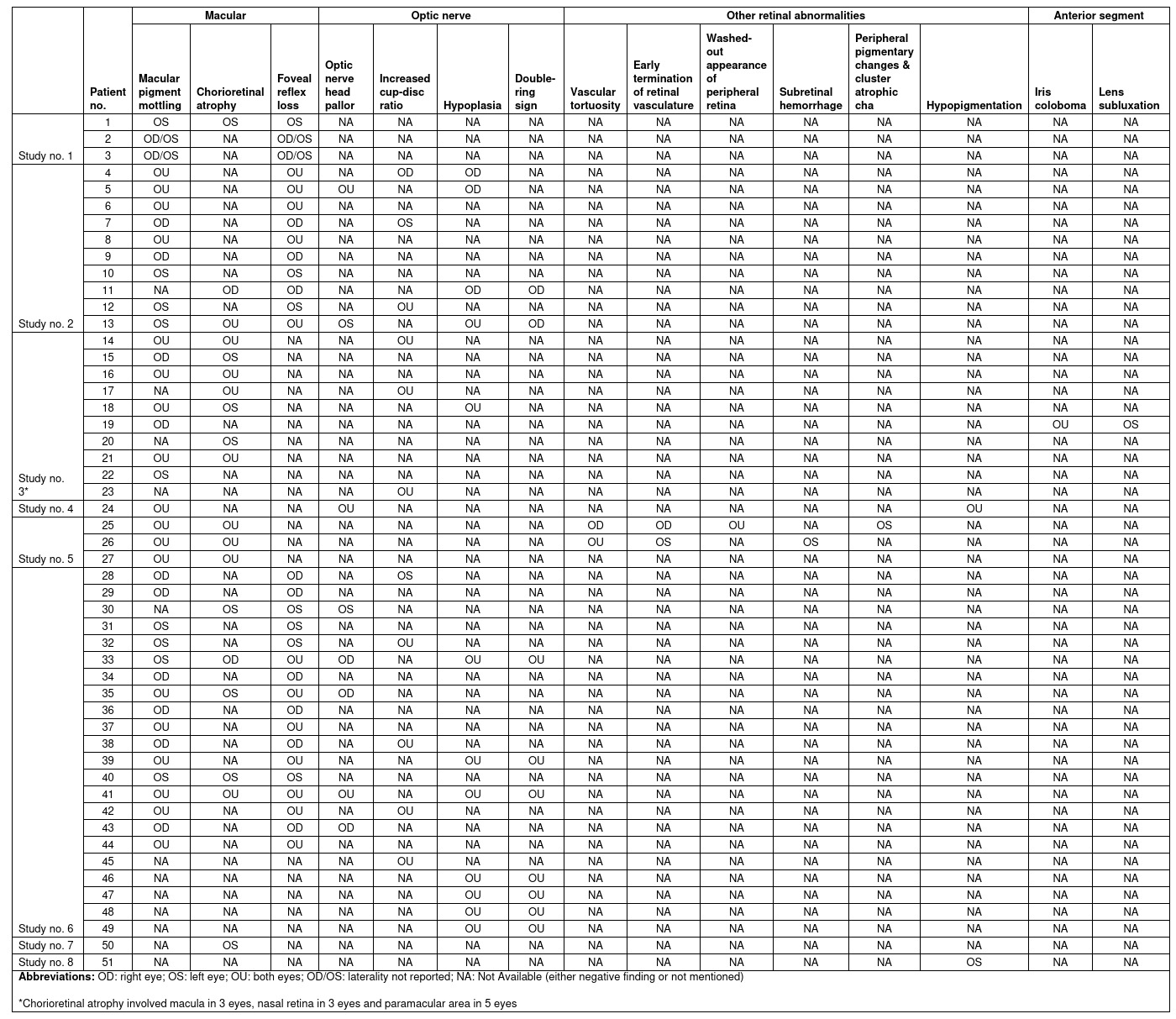
Table 2: List of reported infants with Zika virus related clinical ocular findings.
All 12 included studies were case reports or case series published in either Brazil or United States. 9 studies [9-17] reported the infant cases with a total of 84 eyes in 51 infants, while 3 studies reported the adult cases [18-20] with a total of 5 eyes in 3 adults. Clinical features were reported in 11 studies, whereas ophthalmic imaging findings were reported in 2 studies. Among the 51 infants, 49 had microcephaly. Further subgroup analysis (i.e. infants with microcephaly vs. without microcephaly) was not performed due to small number of cases without microcephaly. Definite Zika virus infection could not be confirmed before February 2016 due to unavailability of the diagnostic tests in Brazil. Among the 9 studies which reported infant cases, 4 studies [9,10,11,13] included cases of presumed Zika virus infection; 3 studies [12,15,16] included cases of confirmed Zika virus infection; and 2 studies [14,17] included both presumed and confirmed cases.
Ocular Abnormalities in Infants
The prevalence of Zika virus related congenital ocular abnormalities remains unknown on literatures. We summarized the findings in 51 infants diagnosed with Zika Virus infection and the associated ocular features in Table 2. Table 3 shows the calculated prevalence of different clinical ocular findings in infants in percentages.
| Clinical ocular findings | No. of studies reporting (n=8) | No. of cases (n=51) | No. of eyes affected (n=84) |
| Macula | |||
| Pigment mottling | 6 | 39 | 57 |
| Chorioretinal atrophy | 6 | 14 | 18 |
| Foveal reflex loss | 3 | 30 | 42 |
| Optic nerve | |||
| Optic nerve head pallor | 3 | 8 | 11 |
| Increased cup-disc ratio | 3 | 11 | 19 |
| Hypoplasia | 3 | 12 | 21 |
| Double‐ring sign | 2 | 9 | 16 |
| Other retinal abnormalities | |||
| Vascular tortuosity | 1 | 2 | 3 |
| Early termination of retinal vasculature | 1 | 2 | 2 |
| Washed‐out appearance of peripheral retina | 1 | 1 | 2 |
| Subretinal hemorrhage | 1 | 1 | 1 |
| Peripheral pigmentary changes and | 1 | 1 | 1 |
| cluster atrophic changes (polar bear tracks) | |||
| Hypopigmentation | 2 | 2 | 3 |
| Chorioretinal atrophy (not macular) | 2 | 5 | 10 |
| Anterior segment Iris coloboma | 1 | 1 | 2 |
| Lens subluxation | 1 | 1 | 1 |
Table 3: Summary of the clinical ocular findings related to Zika virus in infants.
Clinical findings
Macular pigment mottling and foveal reflex loss: Macular pigment mottling and foveal reflex loss are the commonest forms of reported ocular abnormalities, affecting 57 eyes (55.9%) of 39 patients and 42 eyes (41.2%) of 30 patients respectively. Pigment mottling was classified into mild and gross in 3 studies, including study no.1, 2 and 6 by Ventura et al. [9,10,14] Among 38 eyes with pigment mottling in these 3 studies, 20 were mild and 18 were gross.
Chorioretinal atrophy: Chorioretinal atrophy was reported to occur at the macula, paramacular area, and peripheral retina and tend to be well delineated. Predilection for the macular region were demonstrated in 18 eyes (17.6%) of 14 patients, compared to other areas affected in 10 eyes (9.8%) of 5 patients. 2 different forms of chorioretinal atrophy were observed in study no. 3 by Freitas et al [11], the first one is circumscribed areas without visible choroidal vessels and the second one is circumscribed areas of pigmentary clumping. Surrounding hyper-pigmented halos were noted in some cases [11]. Torpedo-shaped atrophic lesion in the macula was demonstrated in one case [13].
Optic nerve abnormalities: Optic nerve abnormalities included optic nerve head pallor (10.8%), increased cup-disc ratio (18.6%), optic nerve hypoplasia (20.6%) and double ring sign (15.7%).
Other retinal abnormalities: Vascular tortuosity (2.9%), early termination of retinal vasculature (2.0%), washed-out appearance (2.0%), subretinal hemorrhage (1.0%) and polar bear tracks (1.0%) were reported in study no. 5 by de Miranda et al as an expanded spectrum of congenital ocular findings [13]. These were not reported in other case series so their percentages were relatively low. Hypopigmentation of peripheral retina (2.9%) was reported in 2 cases [12,16].
Anterior segment: An infant with bilateral iris coloboma and lens subluxation of left eye was reported in study no.3 by Freitas et al. [11] The remaining 7 studies did not report abnormal features in anterior segment.
OCT findings: Optical Coherence Tomography (OCT) was used to look for retinal and choroidal abnormalities of 8 infants with Congenital Zika syndrome (CZS) in study no.9 by Ventura et al. [17] Retinal alterations i.e. chorioretinal scar or pigment mottling were seen in 11 of 16 eyes. OCT was then performed in 9 of the 11 affected eyes. The major OCT findings were discontinuation of the ellipsoid zone and hyper-reflectivity underlying the retinal pigment epithelium, followed by retinal thinning, choroidal thinning and colobomatous-like excavation involving the neurosensory retina, retinal pigment epithelium, and choroid [17].
Ocular Abnormalities in Adults
Non-purulent conjunctivitis was reported in adults with Zika virus infection [21]. It was the commonest type of ocular diseases related to Zika virus, affecting 10-15% of patients.
Uveitis was reported to be a possible manifestation. Furtado et al [19] reported a case who presented with conjunctival hyperemia, keratic precipitates and inflammatory cells in the anterior chamber of the eyes after the onset of Zika virus infection. Zika virus RNA was present in aqueous humor of right eye. [19] Fontes et al reported another case of hypertensive iridocyclitis in a man with presumed Zika virus infection [18]. Unilateral Acute Idiopathic Maculopathy (UAIM) was also reported to be related to Zika virus infection in one adult case [20].
Treatment and prognosis
Currently, there are no vaccine and anti-viral therapy for Zika virus [22,23]. It is mainly managed by symptomatic treatment. In adult cases, topical steroid, antibiotics and cycloplegic agents can be given. Resolution of symptoms have been demonstrated in 2 studies [18,19]. Prognosis of the related ocular problems remains unclear due to unavailability of studies with long term follow-up.
Discussion
Ocular manifestations
We summarized the ocular manifestations of Zika virus infection in 89 eyes of 54 patients. We determined that Zika virus can affect macula, optic nerve, retina and anterior segment. Macular pigment mottling (55.9%), foveal reflex loss (41.2%), chorioretinal atrophy (macular and non-macular) (27.4%), optic nerve hypoplasia (20.6%) and increased cup-disc ratio (18.6%) were the most common ocular findings in infants. The association of iris coloboma and lens subluxation with Zika virus was in question as they only occurred in a single patient [11] but were not seen in other studies. Uveitis, hypertensive iridocyclitis and maculopathy were observed in adult cases.
Prevalence
The prevalence of Zika virus related congenital ocular abnormalities remains unknown. As the first few cases were reported earlier in 2016 [9], there was a lack of appropriate ophthalmologic screening before. Since then, screening guidelines [24] were developed and being adjusted to include more infants with possible Zika virus infection. It is likely that many of the cases were undiagnosed and underreported before the establishment of screening guidelines. We noticed that microcephaly is the primary inclusion criterion in most of the currently available case series (Table 1). However, Zika virus infection does not necessarily lead to microcephaly, and its related ocular abnormalities could also possibly occur in infants without microcephaly [15,16]. Further large-scale studies are required to investigate the prevalence or incidence of different ocular findings regardless of the head circumference.
Diagnosis
In our systematic review, presumed and confirmed cases of Zika virus infection were both included. Before the availability of diagnostic tests, the suspected diagnosis of congenital Zika virus infection was based on the presence of microcephaly in infants, symptoms in mothers during pregnancy and negative screening of other congenital infections. Currently, reverse transcriptase-polymerase chain reaction (RT-PCR) and viral isolation in blood specimens are commonly used to diagnose Zika virus infection, but they are only helpful within 5 days of symptoms onset [25]. On the other hand, Zika virus-specific immunoglobulin M antibodies (ZIKV IgM Ab) can be detected if the suspected cases are tested within 12 weeks [26]. However, interpretation of ZIKV IgM Ab result is limited by its cross-reactivity with other flaviviruses [26,27]. Plaque Reduction Neutralization Test (PRNT) can be used to improve specificity [21].
Apart from blood samples, detection of Zika virus RNA in urine, saliva, conjunctival fluid, breast milk, amniotic fluid, cerebrospinal fluid (CSF), and brain tissue have also been reported [28-34].
Pathogenesis
The issue of whether the reported congenital ocular lesions were a direct result of Zika virus infection or a part of neurodevelopmental defect secondary to microcephaly is still controversial. Microcephaly itself is independently associated with a number of ocular abnormalities, including chorioretinal atrophy, a retinitis pigmentosa clinical picture with spicules and pale nerve, diffuse lacunar chorioretinopathy, retinal pigmentary changes, retinal dysplasia, optic atrophy, optic hypoplasia, optic nerve coloboma, nystagmus, cataracts, micro-ophthalmia, microcornea, falciform folds, esotropia, hyperopia, persistent fetal vasculature, and vascular attenuation [13].
Although some of the ocular findings described previously fit into this microcephaly spectrum, torpedo maculopathy, well delineated macular chorioretinal atrophy and hemorrhagic retinopathy [13] have not been reported in this range. Moreover, ocular lesions have been described in infected infants without microcephaly [15,16] and cellular tropism of Zika virus has been demonstrated in ocular structures [35]. It seems that the ocular abnormalities are not solely caused by microcephaly. Some authors suggested that a dual mechanism that direct infection and the microcephaly related process occurred together was possible [36-38].
The exact mechanisms of how Zika virus invades eyes and visual pathway are still under investigation. A mouse model of ocular diseases [35] confirmed that Zika virus infected eyes of virus-inoculated mice and caused conjunctivitis, panuveitis, and neuroretinitis without global photoreceptor abnormalities. Cellular tropism of Zika virus was evaluated in this model which showed that Zika virus RNA was predominant in retinal pigment epithelium and choroid complex, which was 100-fold higher than that in the optic nerve. The authors suggested that Zika virus might first invade the brain, then the optic tract and migrated along the optic nerve to the eye in a retrograde manner. Another possibility was the hematogenous spread of virus across the blood-retinal barrier. Apoptosis of the optic tract, lateral geniculate nucleus, and the visual cortex have been demonstrated in those mice infected in the postnatal period. It is uncertain whether this process is due to direct damage by the virus or a consequence of inflammatory response.
Congenital ocular abnormalities were not seen in this mouse model. It remains unclear whether those reported human cases were direct results of Zika virus infection or a part of neurodevelopmental defect secondary to microcephaly [35].
Possible Risk Factors for Ocular Findings in Infants
From our systematic review, macular and optic nerve abnormalities were most commonly found in infants with congenital Zika virus infection. A cross-sectional study was conducted by Ventura et al. [14] who suggested that smaller cephalic diameter at birth and mothers with symptoms during the first trimester of pregnancy were the possible risk factors for eye involvement in infants with suspected congenital Zika virus infection. To be more specific, macular alterations were associated with these risk factors (P<0.05) but not optic nerve changes (P=0.35). No specific maternal symptoms were statistically significant when comparing mothers of infants with ocular abnormalities to those without.
Prognosis
As described above, the major manifestations were macular and optic nerve abnormalities which can significantly impair the visual development of infants. Inflammation and scarring of the retina can lead to visual loss. Future long-term studies are required to follow up these patients in order to monitor their visual development and determine possible prognostic factors.
Prevention
The US Centers for Disease Control and Prevention recommends that all pregnant women should not travel to an area with Zika virus infection. A test for Zika virus should be offered to those pregnant women who have traveled to an area with Zika or live in an area with Zika. Before getting pregnant, couples should wait for a period if there is possible exposure via recent travel or unprotected sex with a partner with Zika virus infection. Women should wait at least 8 weeks after symptoms start or last possible exposure while men should wait at least 6 months. People who are living in or frequently traveling to areas with Zika should consider delaying pregnancy [39].
Screening
Currently, the US Centers for Disease Control and Prevention recommends that ocular exam should be part of the initial evaluation for infants with possible Zika virus infection. It should be performed in the hospital before discharge or within 1 month after birth. Repeated ocular exam should be done at age 3 months for those infants with laboratory evidence of Zika virus infection and abnormalities consistent with congenital Zika syndrome [24] (Table 4).
| Initial clinical evaluation and management of infants with laboratory evidence of Zika virus infection or abnormalities consistent with congenital Zika syndrome |
| Consultation with Ophthalmologist for comprehensive eye exam and evaluation for possible cortical visual impairment prior to discharge from the hospital or within 1 month of birth. |
| Outpatient management of infants with laboratory evidence of Zika virus infection, but without abnormalities consistent with congenital Zika syndrome. |
| Referral to ophthalmology for comprehensive eye exam within one month of birth. Perform vision screening and assess visual regard at every well child visit, and refer to ophthalmology for any abnormal findings, or for any parental or provider concerns. |
| Outpatient management of infants with laboratory evidence of Zika virus infection and abnormalities consistent with congenital Zika syndrome |
| Repeat comprehensive ophthalmologic exam at age 3 months, and refer to ophthalmology for any abnormal findings, or for any parental or provider concerns. |
Table 4: US Centers for Disease Control and Prevention recommendations on ocular evaluation for infants with possible congenital Zika virus infection.
Limitations
There were several limitations in this literature review. First, the number of available studies was limited so the sample size was small. Second, there was a lack of standardized reporting system of the ocular findings so same features may be described with different description and some of the features were not mentioned in every study. Also, there were underestimation and it would be difficult to determine the most commonly occurred finding related to Zika virus. Third, there was a lack of large scale studies and follow up to estimate the prevalence of different ocular lesions and visual outcomes in affected infants.
Despite of the above limitations, our systematic review is the most up-to-date review that summarized all currently available data on Zika virus related ocular manifestations and is able to guide further large scale studies.
Conclusion
Macular and optic nerve abnormalities are the major ocular findings in infants. There are no vaccines or anti-viral therapy for Zika virus currently, but preventive measures and screening guidelines have been established. Congenital Zika virus infection is highly associated with ocular abnormalities although the causality and precise mechanism of ocular invasion are still under investigation. Further studies on prevalence and prognosis are of paramount importance for the global public health.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
1. Zanluca C, Melo VC, Mosimann AL, Santos GI, Santos CN, et al. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 110(4): 569-72 (2015).
2. Calvet G, Aguiar RS, Melo AS, Sampaio SA, de Filippis I, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: A case study. Lancet Infect Dis. 16(6): 653-60 (2016).
3. Notes from the field: Evidence of Zika virus infection in brain and placental tissues from two congenitally infected new-borns and two fetal losses-Brazil, 2015. MMWR Morb Mortal Wkly Rep. 65: 159-60 (2016).
4. Transmission of Zika virus through sexual contact with travellers to areas of ongoing transmission-continental United States, 2016. MMWR Morb Mortal Wkly Rep 65: 215-6 (2016).
5. Venturi G, Zammarchi L, Fortuna C, Remoli ME, Benedetti E, et al. An autochthonous case of Zika due to possible sexual transmission, Florence, Italy, 2014. Euro Surveill 21(8): 30148 (2016).
6. Simpson DI. Zika virus infection in man. Trans R Soc Trop Med Hyg. 58: 335-8 (1964).
7. Filipe AR, Martins CM, Rocha H. Laboratory infection with Zika virus after vaccination against yellow fever. Arch Gesamte Virusforsch. 43: 315-9 (1973).
8. Barjas-Castro ML, Angerami RN, Cunha MS, Suzuki A, Nogueira JS, et al. Probable transfusion-transmitted Zika virus in Brazil. Transfusion. 56(7): 1684-8 (2016).
9. Ventura CV, Maia M, Bravo-Filho V, Góis AL, Belfort R. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet. 387(10015): 228 (2016).
10. Ventura CV, Maia M, Ventura BV, Linden VVD, Araújo EB, et al. Ophthalmological findings in infants with microcephaly and presumable intra-uterus Zika virus infection. Arq Bras Oftalmol. 79(1): 1-3 (2016).
11. Freitas BP, Dias JRO, Prazeres J, Sacramento GA, Ko AI, et al. Ocular findings in infants with microencephaly associated with presumed Zika virus congenital infection associated with presumed Zika virus congenital infection in Salvador, Brazil. JAMA Ophthalmol. 134(5): 529-535 (2016).
12. Culjat M, Darling SE, Nerurkar VR, Ching N, Kumar M, et al. Clinical and imaging findings in an infant with Zika Embryopathy. Clin Infect Dis. 63(6): 805-11 (2016).
13. de Miranda HA, Costa MC, Frazaq MAM, Simão N, Franchischini S, et al. Expanded spectrum of congenital ocular findings in microcephaly with presumed Zika infection. Ophthalmology. 123(8): 1788-94 (2016).
14. Ventura CV, Maia M, Travassos SB, Martins TT, Patriota F, et al. Risk factors associated with the Ophthalmoscopic findings identified in infants with presumed Zika virus congenital infection. JAMA Ophthalmol. 134(8): 912-8 (2016).
15. Ventura CV, Maia M, Dias N, Belfort R. Zika: Neurological and ocular findings in an infant without microcephaly. Lancet. 387(10037): 2502 (2016).
16. Ventura CV, Fernandez MP, Gonzalez IA, Rivera-Hernandez DM, Lopez-Alberola R, et al. First travel-associated congenital Zika syndrome in the US: Ocular and neurological findings in the absence of microcephaly. Ophthalmic Surg Lasers Imaging Retina. 47(10): 952-955 (2016).
17. Ventura CV, Ventura LO, Bravo-Filho V, Martins TT, Berrocal AM, et al. Optical coherence tomography of retinal lesions in infants with congenital Zika syndrome. JAMA Ophthalmol. 134(12): 1420-1427 (2016).
18. Fontes BM. Zika virus-related hypertensive iridocyclitis. Arq Bras Oftalmol. 79(1): 63 (2016).
19. Furtado JM, Espósito DL, Klein TM, Teixeira-Pinto T, da Fonseca BA. Uveitis associated with Zika virus infection. N Engl J Med. 375(4): 394-6 (2016).
20. Parke DW III, Almeida DR, Albini TA, Ventura CV, Berrocal AM, et al. Serologically confirmed Zika-related unilateral acute maculopathy in an adult. Ophthalmology. 123(11): 2432-2433 (2016).
21. Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika Virus. N Engl J Med. 374: 1552-1563 (2016).
22. Centers for Disease Control and Prevention (https://www.cdc.gov/zika/symptoms/index.html). (2016).
23. Centers for Disease Control and Prevention (https://www.cdc.gov/zika/prevention/index.html). (2016).
24. Update: Interim Guidance for the Evaluation and Management of Infants with Possible Congenital Zika Virus Infection-United States. (2016).
25. Perkasa A, Yudhaputri F, Haryanto S, Hayati RF, Ma’roef CN, et al. Isolation of Zika virus from febrile patient, Indonesia. Emerg Infect Dis. 22(5): 924-5 (2016).
26. Centers for Disease Control and Prevention. (http://www.cdc.gov/zika/pdfs/zika-mac-elisa-fact-sheet-for-hcp.pdf). (2016).
27. Lanciotti RS, Kosoy OL, Laven JJ, Lambert AJ, Johnson AJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis.14: 1232-9 (2008).
28. Calvet G, Aguiar RS, Melo AS, Sampaio SA, de Filippis I, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: A case study. Lancet Infect Dis. 16(6): 653-60. (2016).
29. Notes from the field: Evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses-Brazil, 2015. MMWR Morb Mortal Wkly Rep. 65: 159-60 (2016).
30. Gourinat AC, O'Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis. 21(1): 84-6 (2015).
31. Musso D, Roche C, Nhan T-X, Robin E, Teissier A, Cao-Lormeau V-M. Detection of Zika virus in saliva. J Clin Virol. 68: 53-5 (2015).
32. Sun J, Wu D, Zhong H, Guan D, Zhang H, et al. Presence of Zika Virus in conjunctival fluid. JAMA Ophthalmol. 134(11): 1330-1332 (2016).
33. Dupont-Rouzeyrol M, Biron A, O’Connor O, Huguon E, Descloux E. Infectious Zika viral particles in breast milk. Lancet. 387(10023): 1051 (2016).
34. Carteaux G, Maquart M, Bedet A, Contou D, Brugières P, et al. Zika virus associated with Meningoencephalitis. N Engl J Med. 374(16): 1595-6 (2016).
35. Miner JJ, Sene A, Richner JM, Smith AM, Santeford A, et al. Zika virus infection in mice causes panuveitis with shedding of virus in tears. Cell Rep. 16(12): 3208-18 (2016).
36. Vasconcelos-Santos DV, Andrade GM, Caiaffa WT. Zika virus, microcephaly, and ocular findings. JAMA Ophthalmol. 134(8): 946 (2016).
37. Moshfeghi DM, de Miranda HA, Costa MC. Zika virus, microcephaly, and ocular findings. JAMA Ophthalmol. 134(8): 945 (2016).
38. Belfort R Jr, de Paula Freitas B, de Oliveira Dias JR. Zika virus, microcephaly, and ocular Findings-Reply. JAMA Ophthalmol. 134(8): 946-7 (2016).
39. Centers for Disease Control and Prevention. (https://www.cdc.gov/zika/pregnancy/women-and-their-partners.html). (2016).